Oxidative stress increases oxidizability of apolipoprotein-B containing lipoproteins and decreases paraoxonase (PON) activity in hemodialysis (HD) patients and plays an important part in the development of atherosclerotic cardiovascular diseases. In HD patients, plasma ascorbic acid (AA) levels are decreased either due to the loss by hemodialysis membranes or due to malnutrition and contribute to the imbalance of antioxidant defense mechanisms. We hypothesized that long-term ascorbic acid (AA) supplementation recovers oxidizability of lipoproteins in HD patients by reinforcing PON activity.
MethodsTwenty-nine adult patients were treated with 100mg and 500mg AA at the end of each HD session thrice a week for two consecutive 16 weeks-periods, respectively. Blood samples were obtained before the first HD session and prior to the first HD sessions following the 100mg AA-supplemented and the 500mg AA-supplemented periods.
ResultsPON activities were significantly increased after 100mg (p<0.05) and 500mg AA (p<0.001) supplementation periods compared to the basal level. Apo-B lipoprotein oxidizability (Δ-MDA) was significantly decreased after 500mg AA supplementation compared to both basal (p<0.05) and 100mg AA supplementation periods (p<0.05). Plasma AA concentrations were negatively correlated with Δ-MDA levels (R=−0.327; p<0.01).
ConclusionOur results suggest that long-term parenteral 500mg AA supplementation improves PON activity alleviating apo B-containing lipoproteins oxidizability in HD patients.
El estrés oxidativo aumenta la susceptibilidad a la oxidación de las apolipoproteínas-B que contienen lipoproteínas y reduce la actividad de paraoxonasa (PON) en pacientes de hemodiálisis (HD) formando un papel importante en el desarrollo de enfermedades arterioescleróticas cardiovasculares. En pacientes de HD, los niveles de ácido ascórbico (AA) plasmático disminuyen debido a la pérdida por membranas de hemodiálisis o por desnutrición, y contribuye al desequilibrio de los mecanismos de defensa antioxidantes. Nuestra hipótesis es que a largo plazo la suplementación con AA recupera la susceptibilidad a la oxidación de las lipoproteínas en pacientes de HD al reforzar la actividad de PON.
MétodosSe trataron 29 pacientes adultos con 100 y 500mg de AA al final de cada sesión de HD/3 veces por semana/durante 2 períodos consecutivos de 16 semanas, respectivamente. Se obtuvieron muestras de sangre antes de la primera sesión de HD y previo a las primeras sesiones de HD luego de los 100mg suplementados con AA y los periodos suplementados con 500mg de AA.
ResultadosLas actividades de PON aumentaron significativamente después de los periodos de suplementación de 100mg (p<0,05) y de 500mg de AA (p<0,001) comparados con el nivel base. La susceptibilidad a la oxidación de la lipoproteína apoB (Δ-MDA) disminuyó significativamente luego de la suplementación de 500mg de AA en comparación con períodos de valores base (p<0,05) y los de 100mg de AA (p<0,05). La correlación entre las concentraciones de plasma AA y los niveles de Δ-MDA resultó negativa (R=−0,327; p<0,01).
ConclusiónNuestros resultados sugieren que la suplementación parenteral a largo plazo de 500mg de AA mejora la actividad de PON mitigando la susceptibilidad a la oxidación de las lipoproteínas que contienen apoB en pacientes en HD.
Chronic kidney disease (CKD) patients have a high prevalence of atherosclerosis and are at higher risk of cardiovascular complications than subjects in the general population.1,2 The risk is even higher in patients undergoing hemodialysis (HD), with cardiovascular mortality accounting for 40% of all-cause mortality in this group.3 Recent reports suggest that factors, other than traditional cardiovascular risk factors are ascribed to the increased prevalence of cardiovascular disease in patients with CKD1 and hemodialysis.3 Several parameters related to inflammation, oxidative stress, and HDL function have increasingly been studied as non-traditional risk factors of atherosclerotic cardiovascular diseases (CVD).4
It is reported that oxidative stress plays an important part in the pathogenesis of atherosclerotic CVD in HD patients.5 HD increases plasma levels of reactive oxygen species (ROS) and causes impairment in total antioxidant status.6–8 The biocompatibility of the dialysis membranes that lead to activation of the immune system is accused of inflammation-induced free radical production in HD patients.9 It is also suggested that oxidative stress may be increased because of a loss of antioxidants during dialysis,10 and malnutrition decreasing the uptake of dietary antioxidants11 in chronic HD patients. The attenuation in antioxidant defense mechanisms and the raised ROS load in HD, expedite oxidative damage to tissues and molecules, particularly cell membranes and lipoproteins that are rich in lipids. This leads to increased vascular oxidative stress which facilitates oxidative modification of lipoproteins that play a key role in the formation of atherogenesis.1,12 Although LDL oxidation has been accepted as the major actor in atherogenesis it is also shown that all apolipoprotein (apo) B-containing lipoproteins (LDL, VLDL, Lp(a) and remnants of chylomicron and VLDL) are retained and then modified in the subendothelial space and thus play role in the initiation and progression of atherosclerosis.13 There is direct evidence that increased plasma concentrations of apolipoprotein-B (apoB)-containing lipoproteins are causatively linked to atherosclerotic CVD.14
It is well-known that HDL can play an important role in the prevention of atherosclerosis and atherosclerotic CVD via mechanisms such as reverse cholesterol transport as well as antioxidant properties.15 Paraoxonase (PON1) is a major antioxidant enzyme that is mostly associated with the HDL particle in serum and is thought to play an important role in HDL mediated antioxidant activity since it protects lipoproteins from oxidation.15 PON1 also inhibits oxidative changes in the HDL that acts to preserve the anti-atherogenic functionality of HDL.16
It is important to evaluate the function of HDL in atherosclerotic CVD, and investigating serum paraoxonase activity in terms of HDL function seems to be one of the practical ways since it is easy to measure in the routine laboratory setting as it can easily be adapted to autoanalyzers. Obviously, PON-1 activity is also a sensitive marker of antioxidant status.17 PON-1 possesses at least two enzymatic activities: PON and Arylesterase (AE). PON hydrolyzes organophosphate compounds such as paraoxon, and AE hydrolyzes carboxylic esters such as phenylacetate. These PON and AE activities establish the antioxidant property of PON-1.18 In patients with CKD, especially in those on long-term hemodialysis therapy, serum PON, and AE activities have been consistently shown to be decreased compared with healthy controls.15,18,19
Several studies have found low PON activity to be a predictor of atherosclerotic CVD.15 Furthermore, recent studies have found that low serum and HDL PON function is associated with significantly worse outcomes in patients with advanced (predialysis) chronic kidney disease.15
Ascorbic acid (AA), another important antioxidant, is a water soluble vitamin that is shown to protect against lipid peroxidation.20 Its action is through scavenging ROS via very rapid electron transfer.21 It is demonstrated that AA intake is associated with an increase in PON activity and that it protects PON-1 against oxidative damage in vitro.22 In CKD, especially in HD patients, plasma AA levels are decreased.23 Either due to the loss by hemodialysis membranes or due to malnutrition, the reduction in plasma AA concentrations contributes to the imbalance of antioxidant defense mechanisms. And, it is documented that patients with lower plasma AA concentrations exhibit a higher risk for cardiovascular events.23
The outstanding role of oxidative stress in the development of CVD has raised interest in the usefulness of antioxidant supplementations in dialysis. Antioxidant supplementation to patients and/or use of antioxidant-coating on HD materials have been widely studied and varying conclusions have been reported on their beneficial effects.23–25 Effect of intravenous or oral AA supplementation in various dosages and durations has been investigated in HD patients and besides opposing reports, the beneficial role of this antioxidant vitamin on oxidative markers and lipid profile has been demonstrated in a great number of studies.25 Although it is well known that AA supplementation diminishes ROS load and restores PON-1 activity,21 the studies about its impact on PON-1 activity in HD patients are restricted.22 Since PON-1 is a crucial enzyme in the prevention of lipoprotein oxidation and atherogenic diathesis, the influence of antioxidant supplementations on its activity must be better defined in dialysis patients.
In their review, Padayatty et al. suggested that future studies of antioxidant actions of vitamin C should target selected patient groups and these groups should be known to have increased oxidative damage as assessed by a reliable biomarker or should have high morbidity and mortality due to diseases thought to be caused or exacerbated by oxidant damage.26 In this study, we hypothesized that long-term supplementation with AA lowers the oxidative stress on lipoproteins and recovers their oxidizability in HD patients, possibly by reinforcing PON-1 activity. Because apo B-containing lipoproteins are retained and modified in the subendothelium, we believe that it would be useful to investigate the oxidative status and oxidizability of apo B-containing lipoproteins to better understand the pathophysiology of atherogenesis in HD patients. Accordingly, in this prospective study, we aimed to evaluate the changes in plasma PON and AE activities, and oxidative status and oxidizability of apo-B containing lipoproteins in HD patients following 16 weeks of AA supplementation in two different doses.
Material and methodsTwenty-nine adult patients, aged between 23 and 67 years, which were on dialysis and erythropoietin (EPO: maintenance doses of recombinant human erythropoietin-alpha or beta) treatment for at least 6 months in the Hemodialysis Unit of Internal Medicine Department in our Medical Faculty, were included in this study. Cigarette smoking, alcohol consumption, antioxidant vitamin supplementation, use of anti-lipemic or non-steroidal anti-inflammatory drugs, or immunosuppressive agents were postulated as exclusion criteria. Patients that were diagnosed with malignancy, hyperparathyroidism or vasculitis, who had B12, B6, or folate deficiency, who had evidence of acute or chronic infections, active inflammatory conditions, hepatic or respiratory diseases, hemoglobinopathies, or had evidence of significant bleeding [decrease in hemoglobin (Hb) level>2g/dL], or had a blood transfusion and change in transferrin saturation (TSAT) (<30 or >50%) and ferritin levels (<100 or >800ng/mL) during the preceding 6 months were not included in the study. The study protocol and the procedures performed in the studies were in accordance with the guidelines of the 1964 Helsinki Declaration and its later amendments and were approved by the local institutional ethics review board (Protocol number 2005-7/14; date of approval: 29 March 2005). All subjects were informed about the procedures of the study and gave specific written informed consent.
There was no change in patients’ dialysis strategy throughout the entire study. The dialyzes were carried out using Fresenius 4008 B device (Fresenius Medical Care, Germany) in all patients with a standard bicarbonate dialysate composition (in mmol/L; bicarbonate 32, acetate 3, Na+ 140, K+ 2, ionized Ca++ 1.5, Mg++ 0.5, chloride 111). All patients had HD three times a week for 4–5h with synthetically modified cellulose (Diacap SMC 1.2, B. Braun, Melsungen, Germany) or haemophan (GFS plus 11, Gambro, Hechingen, Germany) membranes. The haemodialysers were not reused and the dialysis water was obtained from a reverse osmosis treatment system (Aqua RO modular, Fresenius Medical Care, Bad Hamburg, Germany) equipped with an endotoxin filter. It is well known that chloramine contamination in dialysis water can cause hemolysis and use of activated charcoal filters is the best method to eliminate it.27 According to the Dialysis Centers Regulation still in force in our country, dialysis centers use reverse osmosis system in the water system; pre-treatment system consisting of sand filter, activated carbon filter, softener, microparticle trap; and they use the ultraviolet or ultrafilter system for sterilization. The quality of dialysis water was regularly checked according to recommended guidelines. Standard fluid and dietary restrictions (which consisted of 1.2g/kg/day protein, 50mmol sodium, restricted potassium, and phosphate) were applied to all patients and a constant ultrafiltration volume was maintained (maintaining a constant ultrafiltration volume).
All patients were treated with 100mg and 500mg AA (Redoxon ampoule, Bayer Turkish Chemical Industry Trade Co. Ltd., Istanbul, Turkey) in 100mL 0.9% NaCl intravenously, at the end of each dialysis session during the first 16 weeks and the second 16 weeks, respectively. In addition to routine complete blood counts done every month in dialysis patients, blood samples were obtained following overnight fasting before the first HD session (0 week-basal), and prior to the first HD sessions following the 16th (100mg AA-period) and 32nd weeks (500mg AA-period). Blood samples that were drawn into plain, heparinized, and EDTA coated tubes were centrifuged at 1500×g for 10min to obtain serum and plasma specimens. Whole blood counts, ferritin, transferrin saturation (TS), serum urea, total protein, albumin, glucose, total cholesterol (T-chol), HDL-cholesterol (HDL-chol), and triglyceride (TG) measurements were made immediately after blood collection, while aliquots of EDTA plasma were kept at +4°C to evaluate apo-B containing lipoprotein oxidizability, in 24h; serum and plasma samples were stored at −20°C until analyzed for PON and AE activities, Apo AI, Apo B, and AA measurements. To keep hemoglobin levels within the target range (11–12g/dL), minimal changes (increase or decrease) in erythropoietin doses were made if necessary.
AnalysesWhole blood counts, including Hb, haematocrite (Hct) and lymphocyte were evaluated by using Abbott Cell-Dyn 3700SL autoanalyzer (Abbott Laboratories, Diagnostic Division, Illinois, USA) while ferritin levels were determined by using chemiluminescent method (DPC Immulite 2000, Scientific Affairs, DPC Biermann, Germany); serum glucose, total protein, albumin, iron and iron binding capacity (IBC) and urea levels were measured by using Aeroset System Abbott autoanalyzer (Abbott Laboratories, Diagnostic Division, Illinois, USA); TS percentage was calculated according to the formula: TS (%)=Iron/IBC×100. T-chol, TG and HDL-chol were measured by using commercially available Abbott kits in autoanalyzer (Architect ci8200, USA) while Apo AI and Apo B levels were measured by immunonephelometry (Dade Behring Marburg GmbH, Germany). Low-density lipoprotein cholesterol (LDL-chol) concentrations were calculated according to Friedewald's formula28 Oxidative status and oxidizability of apo B-containing lipoproteins were evaluated as described by Zhang et al.29 Oxidizability of lipoproteins were measured by the method depending on the susceptibility of non-HDL fraction to copper-catalyzed oxidation. Oxidative status and oxidizability of apo B-containing lipoproteins were expressed as basal MDA (nmol/mL) and delta-MDA (nmol MDA/mg cholesterol), respectively. Serum PON and AE activities were evaluated by the spectroscopic methods described by Eckerson et al.30 and Haagen and Brock31 and were expressed in terms of “U/L” and “kU/L”, respectively. Plasma AA concentrations were determined according to the spectroscopic method described by Mc Cormick et al.32 and were expressed in mg/dL.
True dialysis times (T), the intradialytic weight losses (UF) and patients’ dry weights were obtained. Adequacy of dialysis (Kt/V) for urea was calculated using the single-compartment model of Daugirdas, standard urea removal ratio using [URR=100 (1−R), where R=post-dialysis urea/pre-dialysis urea], and protein catabolic rate per normalized body weight (nPCR, g/kg/day) using the formula recommended by the DOQI HD Adequacy Work Group.33
StatisticsResults of the measurements in three periods (Basal, 16th week, and 32nd week) were compared to evaluate the effects of treatment with 100mg and 500mg AA. Statistical analysis was performed using statistical software IBM SPSS v.23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and all continuous variables were summarized in terms of means (± standard deviation). Approximate normal distribution was assessed by One-Sample Kolmogorov–Simirnov test. The differences between the three study periods were determined by Multivariate test and Mauchly's tests and were compared using Paired samples T-test and Wilcoxon tests where appropriate. Pearson correlation analysis was performed to test the relationship between AA and Δ-MDA, PON, and AE activities. A value of p<0.05 was considered statistically significant.
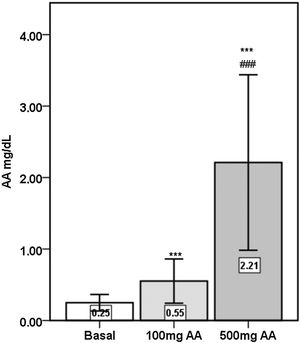
ResultsOf the 29 patients enrolled in the study, 14 were women. The mean age of the study population was 39±11 years, and the mean duration of dialysis prior to the study was 66±55 months. BMI of the patients did not change during the 32-weeks study period (basal=22.4±3.7; 16th week=22.4±3.8; 32nd week=22.4±3.9; p>0.05).
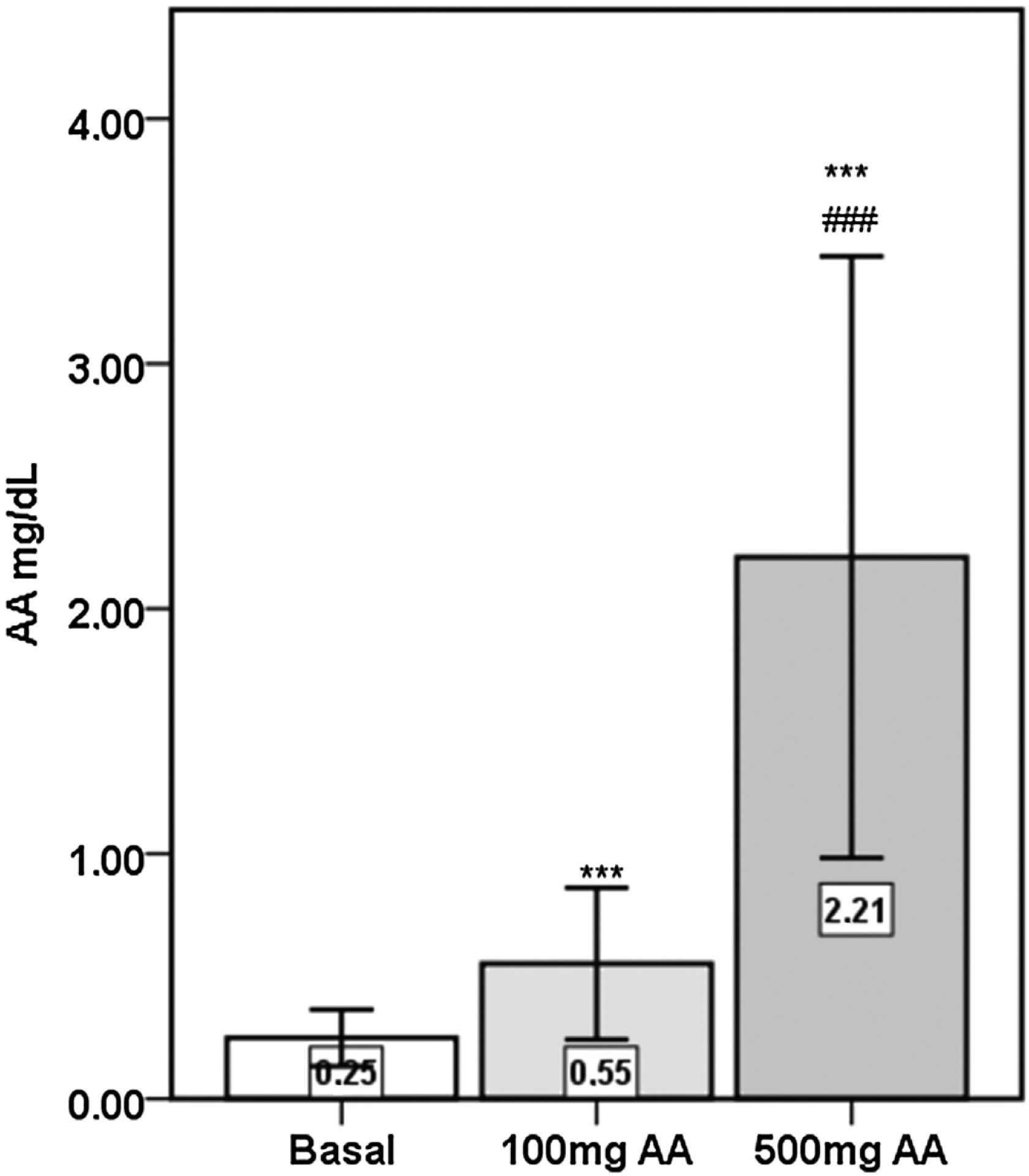
Plasma AA concentrations were increased significantly following the 16-weeks of AA supplementations (p<0.001), with 500mg AA supplementation causing significantly greater elevations compared to 100mg AA supplementation (p<0.001) (Fig. 1).
Whole blood counts, albumin, glucose and urea concentrations were not different between the three periods. The mean hemoglobin levels (g/dL) and erythropoietin doses (U/kg/w) that were 11.4±3.5 and 106.9±56.7, respectively before treatment were changed to 10.5±1.02 and 106.7±44.6 after 100mg AA and 10.3±1.06 and 112.2±42.6 after 500mg AA supplementation, respectively. The changes were not statistically significant (p>0.05). The mean TS (%) and ferritin (ng/mL) levels which were 46±23 and 1081±607, respectively before treatment, changed to 43±20 and 1611±818 after 100mg AA and 42±24 and 1201±566 after 500mg AA supplementation, respectively. The changes were statistically significant in ferritin levels (p<0.001) but not in TS levels. Significant elevations in total protein concentrations were observed in serum samples from the 100mg AA period (7.10g/dL±0.41; p<0.01) and the 500mg AA period (7.04g/dL±0.49; p<0.05) compared to that of basal levels (6.89g/dL±0.48).
The mean Kt/V (dialysis adequacy) and normalized protein nitrogen appearance (nPNA) (g/kg/day) which were 1.81±0.36 and 1.15±0.22 before treatment were changed to 1.86±0.38 and 1.16±0.18, after 100mg AA, and 1.76±0.43 and 1.18±0.20 after 500mg AA, respectively. The changes were not statistically significant (p>0.05).
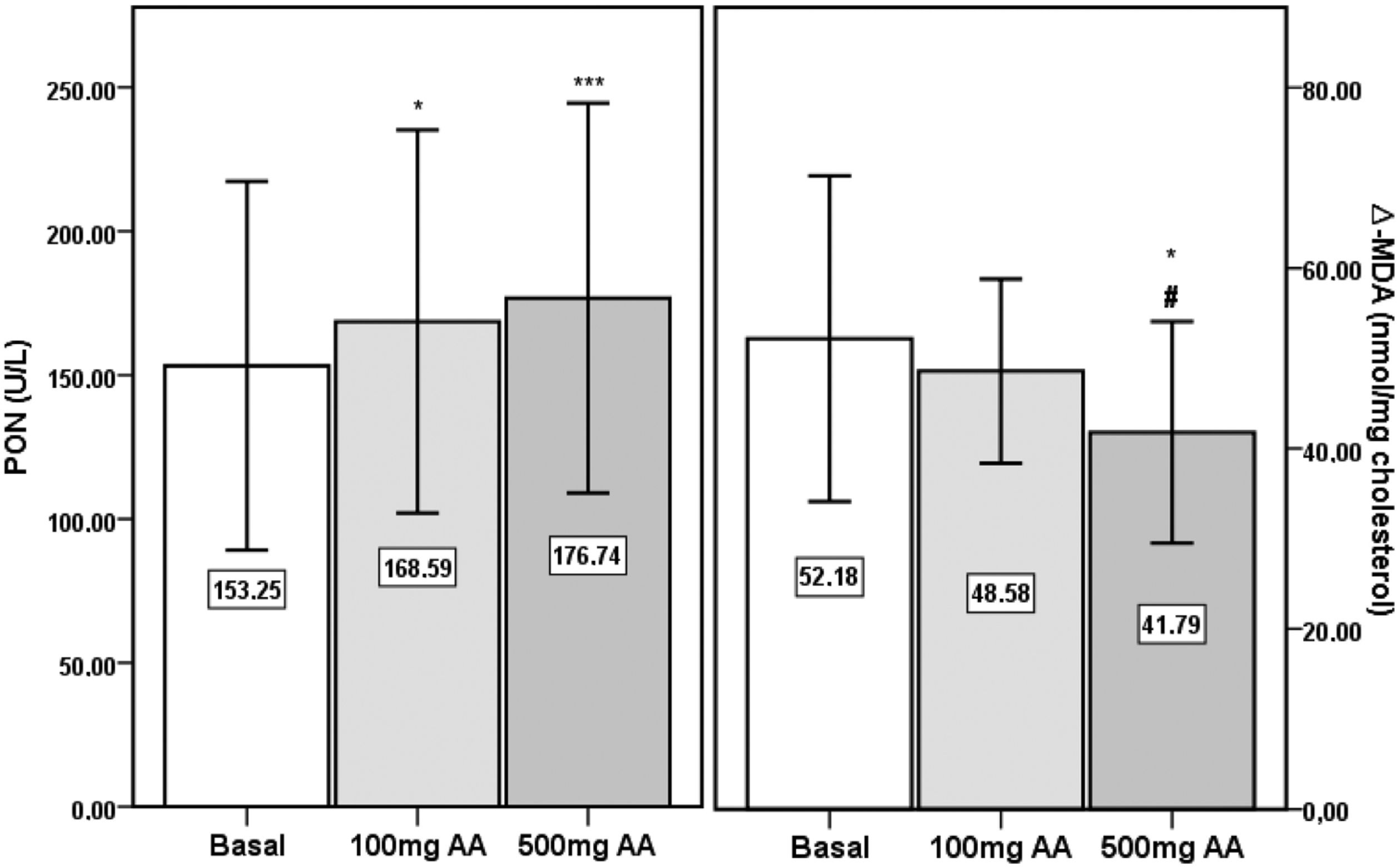
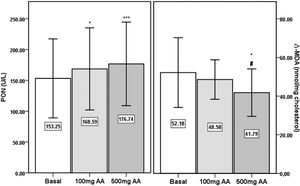
PON activities were significantly increased after 100mg (p<0.05) and 500mg AA (p<0.001) supplementation periods compared to the basal level (Fig. 2). The significance in the elevation in AA supplemented samples persisted following correction of PON activity by HDL-cholesterol (basal: 371U/g±185; 100mg AA: 426U/g±154; 500mg AA: 439U/g±192) (p<0.01). Similarly, PON activities of 100mg (158U/g±58) and 500mg AA (168U/g±77) supplemented samples were significantly higher than the basal level (143U/g±63) after correction by Apo AI (p<0.01 and p<0.001, respectively). AE activity was not different between the three periods (basal: 68.0kU/L±19.9; 100mg AA: 72.0kU/L±18.5; 500mg AA: 62.8kU/L±19.9).
Paraoxonase (PON) activity and Δ-MDA in sera from the basal period, and following 100mg and 500mg AA supplementation periods. *, significantly different from Basal period, p<0.05. ***, significantly different from Basal period, p<0.001. #, significantly different from ‘100mg AA’ period, p<0.05.
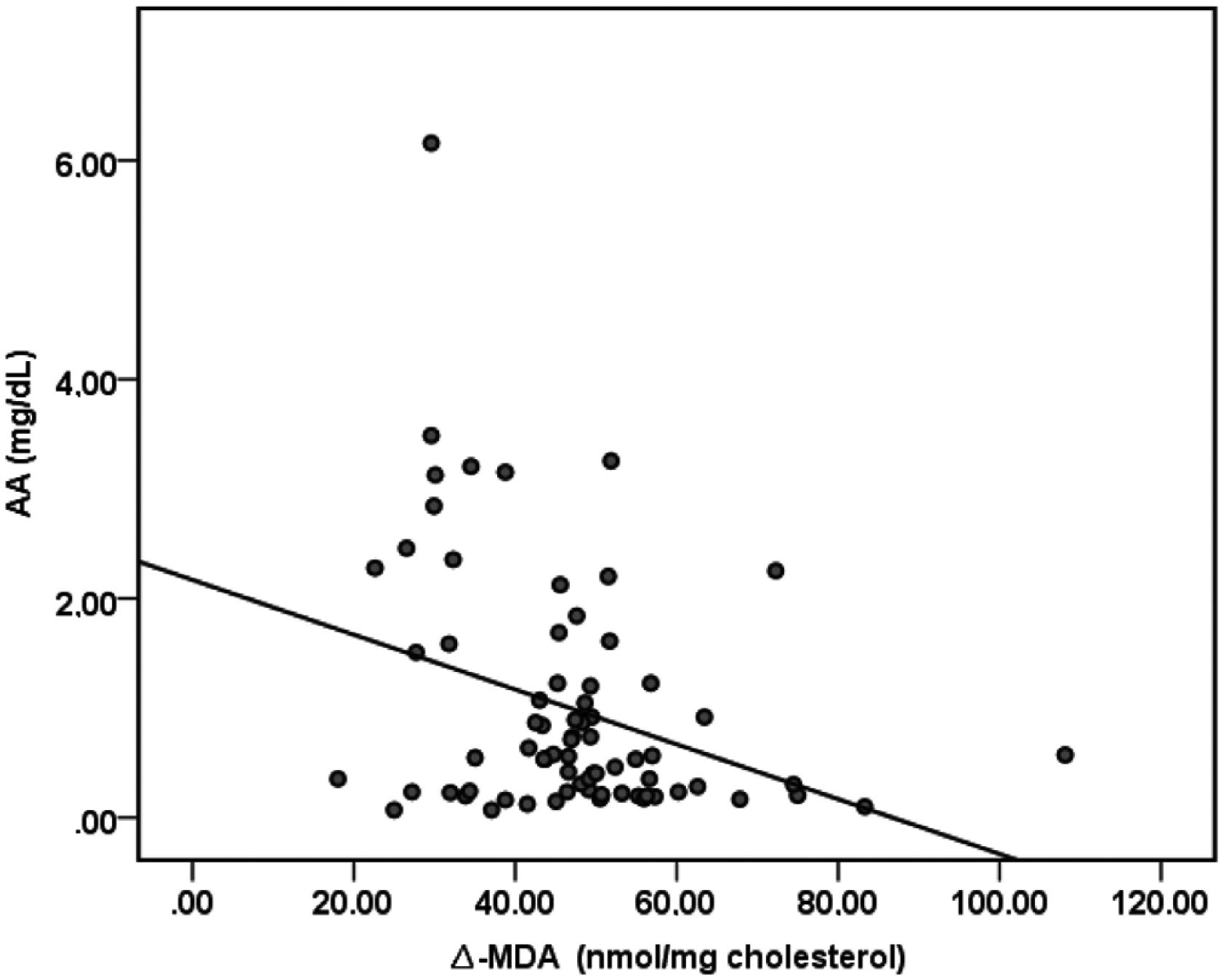
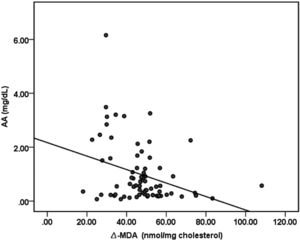
There were no significant differences in MDA concentrations of apo B containing lipoproteins in the three study periods (basal: 7.23nmol/mg chol.±1.06; 100mg AA: 7.45nmol/mg chol.±0.84; 500mg AA: 6.99nmol/mg chol.±1.06). Δ-MDA level in 500mg AA supplementation period was significantly decreased compared to both basal (p<0.05) and 100mg AA supplementation periods (p<0.05) (Fig. 2). Plasma AA concentrations were negatively correlated with Δ-MDA levels (R=−0.327; p<0.01) (Fig. 3).
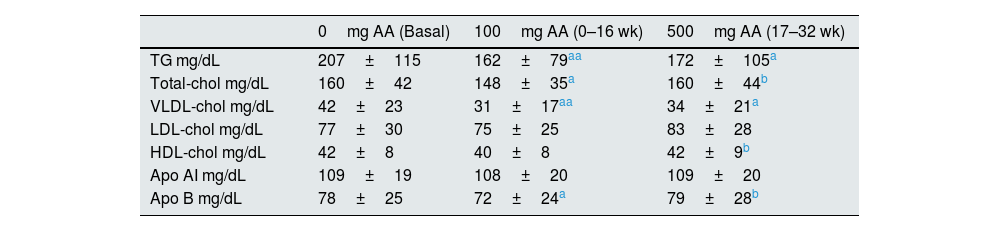
Serum lipid profiles in the three study periods are given in Table 1. TG and VLDL-cholesterol levels were significantly reduced in 100mg and 500mg AA supplementation periods compared to the basal concentrations (p<0.01 and p<0.05, respectively). T-chol and Apo B concentrations were lowered after 100mg AA administration period but the same reduction was not observed after 500mg AA administration period. Serum LDL-chol, HDL-chol, and Apo AI concentrations were not changed from the basal levels after AA supplementation.
Serum lipid and lipoprotein concentrations from the basal period, and following 100mg and 500mg AA supplementation periods.
| 0mg AA (Basal) | 100mg AA (0–16 wk) | 500mg AA (17–32 wk) | |
|---|---|---|---|
| TG mg/dL | 207±115 | 162±79aa | 172±105a |
| Total-chol mg/dL | 160±42 | 148±35a | 160±44b |
| VLDL-chol mg/dL | 42±23 | 31±17aa | 34±21a |
| LDL-chol mg/dL | 77±30 | 75±25 | 83±28 |
| HDL-chol mg/dL | 42±8 | 40±8 | 42±9b |
| Apo AI mg/dL | 109±19 | 108±20 | 109±20 |
| Apo B mg/dL | 78±25 | 72±24a | 79±28b |
AA: ascorbic acid; TG: triglyceride; chol: cholesterol.
The main findings of this survey are that long term (16 weeks-32 weeks) AA supplementation improved PON activity and reduced oxidizability of apo B-containing lipoproteins in HD patients (Fig. 2). It is reported that acetate-based dialysate increases oxidative stress in HD patients.34 Lower basal PON activities and higher basal apo B-containing lipoprotein oxidation may be partly due to the oxidative stress induced by acetate dialysate used in the present study. Since there was no change in patients’ dialysis strategy throughout the entire study, the degree of oxidative stress caused by acetate dialysate was the same in the three periods, and the improvement in oxidative stress markers following treatments indicates the influence of AA supplementation. The improvement in PON activity was more pronounced in 500mg AA supplemented period suggesting that this dosage was more beneficial in enhancing the enzyme activity. The significance in the difference from the basal activity of PON persisted after correction by HDL-chol and Apo AI concentrations, indicating that this difference was independent of the HDL-chol and Apo AI levels (Table 1). AE levels are accepted to reflect the molecular concentrations of PON-1 enzyme.35 Since AE activity was not altered after AA supplementation, we suggest that vitamin C influences merely the enzyme activity, with no effect on enzyme protein metabolism. To our knowledge, there is restricted data about the effect of AA supplementation on PON-1 enzyme activity in HD patients. In 2008, Ferretti et al. have examined the change in PON activity in HD patients after intravenous AA administration.22 In line with our findings, they reported that 500mg AA administration thrice a week increased PON activity significantly after 6 months.
In an early study, Mackness et al. have reported that PON-1 prevents accumulation of lipid peroxidation products in LDL.36 Parallel with their report, Rosenblat et al. have described that PON-1 inhibits Cu-induced LDL-oxidation.37 It is documented that PON-1 protects lipids in lipoproteins from oxidation and that PON-1 possesses peroxidase-like activity that can contribute to its protective effect against lipoprotein oxidation.38 In the present study, 500mg AA supplementation, thrice a week, caused significant reduction in oxidizability of apo B-containing lipoproteins in an opposite pattern from that seen in the improvement of PON activity. The significant negative correlation between plasma AA concentration and Δ-MDA levels (R=−0.327; p<0.01) reveals the protective effect of AA, supporting our hypothesis that AA recovers oxidizability of apo B-containing lipoproteins in HD patients (Fig. 3). In light of the previous reports about the ability of PON-1 on protecting lipoproteins from oxidation, our results suggest that the improvement in PON activity alleviated apo B-containing lipoproteins oxidizability in HD patients. It is well known that oxidation to lipoproteins increases in HD sessions.25,39 This is postulated as one of the most important reasons for the development of CVD in CKD patients. According to our findings, 16 weeks of 1500mg/week AA administration reduces oxidizability of apo B-containing lipoproteins, the primarily responsible lipoproteins in CVD development.
Researches investigating the athero-protective dosage of AA administration in HD-induced oxidative stress have not established a final conclusion.20 The protective effects of AA were blunted in some investigations due to the theoretical concerns about the pro-oxidant nature of high-dose vitamin C.25,40 In the present study (a non-significant) reduction in basal MDA concentration of apo B containing lipoproteins were observed after 16 weeks of 500mg AA administration period, but a statistical significance was not established. However, the MDA concentrations in these lipoproteins being not increased, suggest that AA supplementation, in the dosages administered in our study, did not cause oxidative damage in these lipoproteins. Our results suggest that HD patients may benefit from long term supplementation of 500mg AA thrice a week in protection of lipoprotein-oxidation without giving rise to an oxidative impulse. There are conflicting reports about the effect of AA supplementation on lipoprotein-oxidizability.41,42 Lack of a standard dosage, duration, or way of administration in these studies is probably the reason for the discrepancies between the results. Ramos et al. studied the effect of one year-daily oral supplementation with 1000mg AA on lipid and lipoprotein oxidation in hemodialysis patients.43 They reported that AA significantly abated the hemodialysis-induced increase of MDA concentration in LDL. In the present study, a significant difference was not demonstrated in basal apo B-MDA levels after AA administration. The longer duration of their study and the difference in the way of AA administration may be the reason for the discrepancy between the two studies. In their study, in vitro oxidizability of LDL was evaluated by monitoring conjugated diene production, and contrary to our results, they did not observe a significant improvement. The methodological difference in evaluating the susceptibility of lipoproteins to in vitro oxidation and investigating only the LDL fraction appears to be the cause of this disagreement.
The lipid profile was not altered after AA supplementation in the study by Ramos et al.41 Similar to their findings, serum LDL-chol and HDL-chol concentrations were not changed in the present study. However, in our study, decrements in TG and VLDL-cholesterol levels were observed after AA supplementation. There are different results about the influence of AA supplementation on lipid profile in HD patients. El Mashad et al. demonstrated that 12 weeks of 250mg intravenous AA supplementation 3 times a week, improved lipid profile significantly in children on HD.44 Abdollahzad et al. reported a significant reduction in serum levels of T-chol and LDL-chol in their study where the study group consumed daily 250mg AA, for 12 weeks; however, serum TG and HDL-chol levels were not changed significantly.45 De Vriese et al. demonstrated that plasma lipids were not altered after 360 and 1500mg/week oral AA administration in HD patients.46 The differences may be due to the diversity in the subject profiles, AA dosages, the way, and duration of administration in the surveys.
AA supplementation provided several other benefits in HD patients in the present study. Total protein concentrations were improved by AA supplementation. The essential function of ascorbic acid as a co-substrate in various steps of amino acid and protein synthesis is well known. The improvement in total protein levels may be due to the reinforce AA supplementation provided to protein synthesis, decelerating the protein turnover rate. Protein concentrations of 100mg and 500mg AA periods were not significantly different, indicating that 500mg AA supplementation did not cause additional recovery.
Although significant elevations were observed in total protein levels, AA supplementation did not affect serum albumin levels significantly. Our results are following that of Dae Woong Kang et al., where albumin concentrations were not altered after 500mg AA treatment, three times a week, for 3 months in HD patients with normoferritinemic anemia.47 Albumin is a negative acute phase protein and decreases due to inflammatory conditions. Chronic hemodialysis causes inflammatory response and decreases serum albumin levels and according to the results of this study, neither 100mg nor 500mg AA supplementation for 16 weeks could make a significant improvement in albumin concentrations.
There are several limitations in the present work. First of all, the lack of a control group to compare the basal parameters of HD patients with, restricts the discussion on HD-induced changes in the study population. Previous reports declaring the effect of HD on the parameters discussed were consulted, instead. The second restriction is the small number of the study population. This is due to the strict patient selection criteria of the study. Although examining the same group of patients in three different conditions is a strong point of this study, a group with a larger size would provide better statistical evaluation and higher statistical power. Another point is that a wash-up period between the two supplementation periods was not established. This limits the discussion about the effects of 100mg and 500mg AA supplementation periods. Finally, the lack of data on magnesium (Mg) levels is a restriction. In hemodialysis patients, reduced serum Mg levels have been associated with increased oxidative stress.48 Mg levels were not tracked through out the study period. Data providing the changes in Mg concentrations would enrich the discussion.
ConclusionsIn conclusion, long term parenteral 500mg vitamin C supplementation three times a week, prevents apolipoprotein B containing lipoproteins from oxidation, and reinforce PON activity in HD patients. There is limited data on the influence of vitamin C treatment on plasma PON and AE activities in HD patients, and further studies with different dosages and periods of administration would provide new aspects in potential benefits of vitamin C in the prevention of atherosclerotic changes in HD patients.
Conflict of interestNone.
This work was supported by the Research Fund of The University of Bursa Uludag Project number T-2006/14.