The cardiovascular risk has been increased in chronic kidney disease associated with chronic inflammation and atherosclerosis. Decoy receptor 3, is a member of the TNF receptor superfamily and associated with inflammation and atherosclerosis. The aim of our study is to determine the relationship, between serum DcR3 levels and inflammatory markers in patients with renal transplantation, those receiving dialysis treatment and cases with chronic renal failure that did not receive replacement therapy, and to evaluate their correlation with USG findings.
Material and methodsA total of 150 patients aged between 22–86 years, consisting of 4 groups, namely renal transplantation, dialysis, predialysis chronic kidney disease and control groups, were included in the study. Serum decoy receptor 3, VCAM-1, ICAM-1 and IL-8 measured with ELISA method. Carotid intima-media thickness and presence of carotis arter plaque performed by ultrasound probe, non-invasively.
ResultsAll serum markers were higher in dialysis and pre-dialysis chronic kidney disease groups compared to renal transplant and control groups (p<0.05). Serum decoy receptor 3 level (median(min–max)) of renal transplant group (0.49ng/mL (0.19–1.65)) was higher than control group (0.35ng/mL (0.19–2.22)). There was no difference between patients receiving dialysis (0.89ng/mL (0.41–4.98)) and patients with pre-dialysis chronic kidney disease (0.71ng/mL (0.29–1.68)). There was no difference between patient groups in terms of the presence of plaque.
ConclusionAlthough renal transplantation provides a significant improvement in the inflammatory process, not return completely. Inflammatory process associated with uremic milieu may predispose to atherosclerosis in patients with pre-dialysis chronic kidney disease and hemodialysis patients.
El riesgo cardiovascular se ha incrementado en la enfermedad renal crónica asociada con la inflamación crónica y la ateroesclerosis. El decoy receptor 3 (DcR3) es un miembro de la superfamilia de receptores de TNF y está asociado con inflamación y ateroesclerosis. El objetivo de nuestro estudio es determinar la relación entre los niveles séricos de DcR3 y los marcadores inflamatorios en pacientes con trasplante renal, los que reciben tratamiento de diálisis y los casos con insuficiencia renal crónica que no recibieron terapia sustitutiva, y evaluar su correlación con los hallazgos de la ultrasonografía (USG).
Material y métodosSe incluyeron en el estudio un total de 150 pacientes con edades comprendidas entre los 22 y los 86 años. Así, hay 4 grupos, que en concreto son: trasplante renal, diálisis, enfermedad renal crónica prediálisis y grupos de control. Suero DcR3, VCAM-1, ICAM-1 e IL-8 fueron medidos con el método ELISA. Espesor de la íntima-media carotídea y presencia de placa de la arteria carótida fue realizada por sonda ecográfica de forma no invasiva.
ResultadosTodos los marcadores séricos fueron más altos en diálisis y enfermedad renal crónica previa a la diálisis grupos en comparación con los grupos de control y de trasplante renal (p<0,05). Nivel de DcR3 en suero (mediana [min-máx]) del grupo de trasplante renal (0,49ng/ml [0,19-1,65]) fue mayor que el grupo de control (0,35ng/ml [0,19-2,22]). No hubo diferencia entre los pacientes que recibieron diálisis (0,89ng/ml [0,41-4,98]) y los pacientes con enfermedad renal crónica prediálisis (0,71ng/ml [0,29-1,68]). No hubo diferencia entre los grupos de pacientes en cuanto a la presencia de placa.
ConclusiónAunque el trasplante renal aporta una mejora significativa en el proceso inflamatorio, no vuelve a su estado normal por completo. El proceso inflamatorio asociado con el medio urémico puede predisponer a la ateroesclerosis en pacientes con enfermedad renal crónica previa a la diálisis y pacientes en hemodiálisis.
The incidence of chronic kidney disease (CKD) in Turkey as well as all over the world is gradually increasing. Patients who reach the end-stage renal disease (ESRD) (Glomerular Filtration Rate (GFR)<15ml/min/1.73m2) can only maintain their lives with one of the renal replacement therapies such as hemodialysis (HD), peritoneal dialysis (PD) or kidney transplantation.1
Atherosclerotic cardiovascular diseases are among important causes of mortality and morbidity in patients receiving renal replacement therapy.2 Although cardiovascular diseases (CVDs) are among important causes of mortality even after transplantation, oxidative stress, inflammation and atherosclerosis persist in renal transplant patients who do not have CVD, and greater improvement is observed in these cases compared to HD and PD patients.3,4 Furthermore, as a remarkable finding, cardiovascular and cerebrovascular diseases rank on top among causes of HD-related mortality in Turkey.5
Different inflammatory mediators such as adhesion molecules, chemokines and cytokines play a role in the process of atherosclerosis.6 Especially in patients with ESRD, systemic concentrations of pro-inflammatory and anti-inflammatory cytokines increase several times due to their decreased renal clearance and increased production. Different causes such as dialysis and infection, comorbidities, genetic factors and diet are responsible for persistent inflammation.7 In addition, deterioration of the vascular structure and decrease in nitric oxide (NO) synthesis together with the decrease in renal function accelerate the process of endothelial dysfunction and atherosclerosis.
Asymmetric dimethyl arginine (ADMA) is an analog of L-arginine and contributes to oxidative stress by inhibiting endogenous NO synthase. Plasma ADMA concentration increases with the decrease in GFR. Even in ESRD, ADMA levels can increase 2–3 times of normal values.8 Increased plasma ADMA levels in these patients is an important predictor of cardiovascular death.9
Decoy receptor 3 (DcR3) is a member of the tumor necrosis factor-α (TNF-α) receptor family and is also known as tumor necrosis factor receptor superfamily membran 6B(TNFRSF6B). DcR3 gene is located on the long arm of chromosome 20. It contains 300 amino acids and has a molecular weight of about 35kDa. It contains 4 consecutive cysteine rich regions at its aminoterminal end. DcR3, an antiapoptotic soluble receptor, plays a role in immune modulation.10 DcR3 binds to some members of the TNF family, such as the Fas ligand, inhibiting the biological activities of these molecules.10 DcR3 binds to FAS ligand, LIGHT(TNFSF14) and TNF like molecule 1a(TL1A/TNFSF15), inhibiting the biological activities of these molecules.11 Since these molecules with which it interacts play a role in apoptosis and inflammation response of immune cells, DcR3 has both immune suppressor and pro-inflammatory properties.11 DcR3 also increases the release of molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), interleukin-8 (IL-8) from endothelial cells, and higher levels of these molecules are associated with the risk of CVD.12 Carotid intima-media thickness (CIMT) is a reliable marker used in determining atherosclerosis and it is evaluated by ultrasonography (USG).13 In addition, it has been stated that three-dimensional evaluation of carotid artery plaque volume with USG is more sensitive and reproducible than one-dimensional evaluations.14
The aim of our study is to determine the relationship, between serum DcR3 levels and inflammatory markers in patients with renal transplantation, those receiving HD treatment and cases with chronic renal failure (CRF) that did not receive replacement therapy, and to evaluate their correlation with radiological indicators.
Material and methodsThe research was designed as a cross-sectional study and ethical approval was obtained with the decision of the Meram Medical Faculty Ethics Committee. A total of 150 patients aged between 22 and 86 years, consisting of 4 groups, namely renal transplantation, dialysis, predialysis CRF and control groups were included in the study. The patient group was composed of volunteer patients who came to Meram Medical Faculty Nephrology and Transplantation outpatient clinics for routine control and gave their verbal and written consent. At least 6 months after transplantation patients were included in the renal transplantation group. Stage 2–4 CKD patients with normal urine output were included in the predialysis CRF group. CKD stage was evaluated according to the criteria of GFR Modification of Diet in Renal Disease (MDRD). (MDRD=175×SCr−1.154×age−0.203×0.742 (for female)×1.212 (for black race) ((SCr=Serum creatinine).15 Patients who applied to the Internal Medicine outpatient clinic of Meram Faculty of Medicine for various reasons and did not have any abnormality in their physical examination and laboratory tests were included in the control group.
Patients with urgent renal problem, cardiovascular event, stroke, uncontrolled hypertension, complaints suggesting any infectious disease, any pathologic examination findings and emergency medical conditions. known cardiovascular disease and those using supplements that affect inflammation parameters were excluded from the study.
Biochemical evaluationFollowing an overnight fast, morning blood samples were taken into a gel tube without anticoagulant and centrifuged at 1500g for 10min after the coagulation process was completed. After performing the analyzes routinely requested by the attending physician(s), the remaining serum samples were portioned and stored at −80°C until the day of analysis. Complete blood counts routinely requested for all patients, were performed using samples taken into an EDTA tube. Serum DcR3 (Biovendor, Czech Republic), IL-8 (Boster, USA), VCAM-1 (Boster, USA) and ICAM-1 (Boster, USA) tests were performed using ELISA method. Serum CRP, urea, creatinine, total cholesterol, alanine aminotransferase (ALT) and LDL cholesterol levels were measured by spectrophotometric method (Abbott Architect c16000, Illinois, USA). Complete blood counts were performed with an automated cell counter device (Mindray BC-6800, Shenzhen, PRC) using laser-based flow cytometric impedance method. Whole blood erythrocyte sedimentation rate was determined using fully automated ESR analyzer (iSed, Alcor Scientific).
Radiological evaluationCIMT and plaque volume measurements in all patients and the control group were performed by a single experienced investigator. Carotid arteries were measured with an Applio XG ultrasonography device (Toshiba Medical Systems, Tokyo, Japan) using a 10-MHz linear probe. Average of three measurements made from each side was calculated and recorded as right and left CIMT. CIMT was measured from the thickest places of the carotid artery as much as possible. The presence of plaque was evaluated by ultrasonographic measurements made in the transverse and craniocaudal planes.
Statistical analysisStatistical analysis was performed using SPSS version 20.0 (IBM, USA). The fitness of the data to normal distribution was investigated using the Kolmogorov–Smirnov test. In our study, the data that fit the normal distribution of the groups were given as X±SD, and the results that did not fit were expressed as median (minimum–maximum) values. One-way ANOVA and Tukey tests were used for normally distributed data. Kruskal–Wallis and Mann–Whitney U tests were used as nonparametric tests. Pearson and Spearman correlation tests were used to evaluate the correlation between parameters. Statistical significance was accepted as P<0.05.
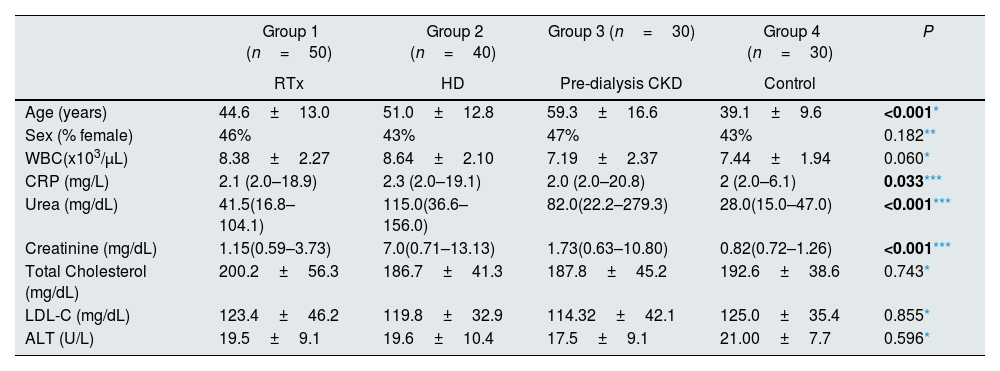
ResultsA total of 150 individuals took part in the study. There was no difference between the groups in terms of gender, WBC, lipid profile and ALT levels (Table 1). The mean age of the patients in the dialysis and predialysis CRF groups was higher than that of the control group, and it was significantly higher in the transplanted group relative to the predialysis group. Serum urea and creatinine levels were the highest in the dialysis group, while their levels decreased gradually in predialysis CRF, transplantation and control groups. There was a significant difference between all groups in terms of serum urea and creatinine levels. Serum CRP levels were significantly higher in the transplantation group compared to predialysis CRF and control groups. The CRP levels of the control group were below 10mg/L (Table 1).
Demographic data and routine biochemical analysis (n=150).
| Group 1 (n=50) | Group 2 (n=40) | Group 3 (n=30) | Group 4 (n=30) | P | |
|---|---|---|---|---|---|
| RTx | HD | Pre-dialysis CKD | Control | ||
| Age (years) | 44.6±13.0 | 51.0±12.8 | 59.3±16.6 | 39.1±9.6 | <0.001* |
| Sex (% female) | 46% | 43% | 47% | 43% | 0.182** |
| WBC(x103/μL) | 8.38±2.27 | 8.64±2.10 | 7.19±2.37 | 7.44±1.94 | 0.060* |
| CRP (mg/L) | 2.1 (2.0–18.9) | 2.3 (2.0–19.1) | 2.0 (2.0–20.8) | 2 (2.0–6.1) | 0.033*** |
| Urea (mg/dL) | 41.5(16.8–104.1) | 115.0(36.6–156.0) | 82.0(22.2–279.3) | 28.0(15.0–47.0) | <0.001*** |
| Creatinine (mg/dL) | 1.15(0.59–3.73) | 7.0(0.71–13.13) | 1.73(0.63–10.80) | 0.82(0.72–1.26) | <0.001*** |
| Total Cholesterol (mg/dL) | 200.2±56.3 | 186.7±41.3 | 187.8±45.2 | 192.6±38.6 | 0.743* |
| LDL-C (mg/dL) | 123.4±46.2 | 119.8±32.9 | 114.32±42.1 | 125.0±35.4 | 0.855* |
| ALT (U/L) | 19.5±9.1 | 19.6±10.4 | 17.5±9.1 | 21.00±7.7 | 0.596* |
RTx: renal transplantation, HD: hemodialysis, CKD: chronic kidney disease, WBC: white blood cells, CRP: C-reactive protein, LDL-C: low density lipoprotein cholesterol, ALT: alanine aminotransferase.
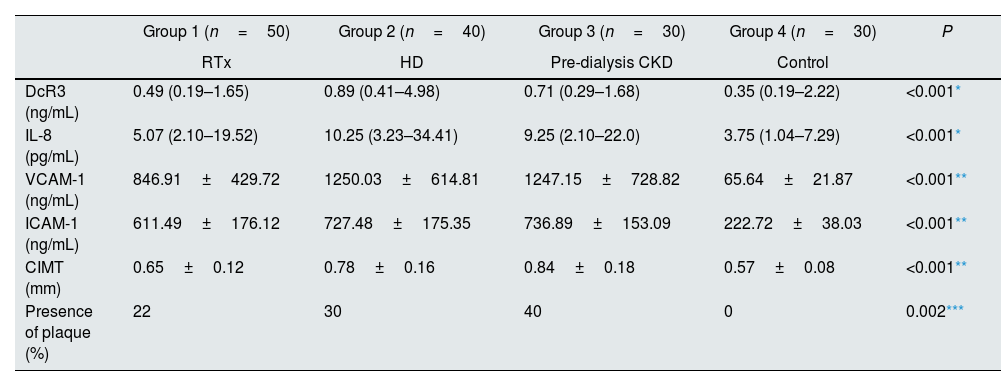
Serum levels of DcR3, IL-8, VCAM-1, ICAM-1 in all three groups were found to be significantly higher than the control group (Table 2). When the patient groups were evaluated among themselves, the DcR3, IL-8, VCAM-1, ICAM-1 levels of the transplanted group were found to be significantly lower than the dialysis and predialysis CRF groups. Any significant difference was not found between the dialysis and predialysis CRF groups (Table 2). When the groups included in our study were examined in terms of age, a significant difference was found between transplantation and predialysis CRF groups, and the mean age of the dialysis and predialysis groups was higher than the control group.
Serum DcR3, IL-8, VCAM-1 ve ICAM-1 levels, radiological findings (n=150).
| Group 1 (n=50) | Group 2 (n=40) | Group 3 (n=30) | Group 4 (n=30) | P | |
|---|---|---|---|---|---|
| RTx | HD | Pre-dialysis CKD | Control | ||
| DcR3 (ng/mL) | 0.49 (0.19–1.65) | 0.89 (0.41–4.98) | 0.71 (0.29–1.68) | 0.35 (0.19–2.22) | <0.001* |
| IL-8 (pg/mL) | 5.07 (2.10–19.52) | 10.25 (3.23–34.41) | 9.25 (2.10–22.0) | 3.75 (1.04–7.29) | <0.001* |
| VCAM-1 (ng/mL) | 846.91±429.72 | 1250.03±614.81 | 1247.15±728.82 | 65.64±21.87 | <0.001** |
| ICAM-1 (ng/mL) | 611.49±176.12 | 727.48±175.35 | 736.89±153.09 | 222.72±38.03 | <0.001** |
| CIMT (mm) | 0.65±0.12 | 0.78±0.16 | 0.84±0.18 | 0.57±0.08 | <0.001** |
| Presence of plaque (%) | 22 | 30 | 40 | 0 | 0.002*** |
RTx: renal transplantation, HD: hemodialysis, CKD: chronic kidney disease, DcR3: decoy receptor 3, VCAM-1: vascular cell adhesion molecule-1, ICAM-1: intercellular adhesion molecule-1, CIMT: carotid intima-media thickness.
The mean CIMT values of HD and predialysis CRF groups were significantly higher than the control and transplantation groups. There was no significant difference between the CIMT values of the dialysis and predialysis CRF groups. When 0.82mm was taken as the threshold value and the groups were evaluated in this respect, it was seen that CIMT values of all patients in the control group were below the threshold value and the percentage of patients who exceeded the threshold value was the highest in predialysis CRF group.16 Groups were compared according to the CIMT threshold value (Table 3).
Comparison of groups according to CIMT threshold value (n=150).
| Group 1 (n=50) | Group 2 (n=40) | Group 3 (n=30) | Group 4 (n=30) | P | |
|---|---|---|---|---|---|
| RTx | Dialysis | Pre-dialysis CKD | Control | ||
| ≤0.82mm | 44 (%88) | 31 (%77) | 15 (%50) | 30 (%100) | * |
| >0.82mm | 6 (%12)a | 9 (%23)b | 15 (%50)c | 0 |
RTx: renal transplantation, HD: hemodialysis, CKD: chronic kidney disease.
No plaque was detected in the control group. The percentage of plaque presence in the dialysis and pre-dialysis CRF groups was higher than in group 1, but this difference was not statistically significant (P>0.05).
In the correlation analysis, significant correlation was found between ICAM-1 and VCAM-1 in the dialysis and pre-dialysis CRF groups (r=0.372, P=0.018 and r=0.403, P=0.027 for the dialysis and pre-dialysis groups, respectively). In addition, there was significant correlation between DcR3 and CIMT in the dialysis group (r=0.328, P=0.039) and between DcR3 and ICAM-1 (r=0.368, P=0.045) in the pre-dialysis CRF group.
DiscussionIn this study, the change in DcR3 levels and the relationship of DcR3 with biochemical and radiological inflammatory markers in CRF patients who underwent renal transplantation, or received HD treatment and CRF patients that did not receive replacement therapy were evaluated. While DcR3, VCAM-1, ICAM-1, IL-8 levels and CIMT values were found to be relatively higher in dialysis and predialysis CRF groups, a significant decrease was observed in the transplanted group compared to the dialysis and predialysis CRF groups.
In addition, serum levels of DcR3, VCAM-1, ICAM-1 and IL-8 in the transplanted group were significantly higher relative to the control group. There is evidence in the literature that the uremic environment seen in CKD causes endothelial dysfunction.17 In addition, it has been stated that cell adhesion molecules such as VCAM-1 and ICAM-1 play an important role in the process leading to atherosclerosis.18 Increased levels of circulating cell adhesion molecules in many diseases have been associated with cardiovascular diseases.18,19 In a study in which CRF patients were evaluated in terms of inflammation and CVD, it was determined that the ICAM-1 levels were significantly increased in CRF patients with CVD, and a strong correlation between ICAM-1 and CRP was noted. In the same study, ICAM-1 and VCAM-1 were associated with mortality and VCAM-1 levels were shown to increase in conjunction with lipoprotein (a) levels.20
IL-8 is another marker associated with atherogenesis.21 IL-8, which plays a role in leukocyte migration, has been found to be associated with coronary artery calcification and atherosclerotic plaque development.22 Moreover, it is seen as an independent risk factor for all causes of long-term mortality in acute coronary syndromes.23 In the study conducted by Weber et al., It was shown that IL-8 and IL-18 levels were correlated with coronary artery calcification in stage 3 and stage 4 CRF patients.22 In our study, similar to the literature, we observed that VCAM-1, ICAM-1 and IL-8 levels were significantly higher in the dialysis and CRF groups compared to the control and the transplant groups.
There was no significant difference between the dialysis and CRF groups. This may suggest that the inflammatory process triggered by the uremic environment and the inhibition of the excretion of these molecules together with the decrease in renal clearance may cause an increase in the levels of adhesion molecules. In a study conducted with patient groups similar to our study,24 VCAM-1 and ICAM-1 were found to be significantly higher in the HD group than both the control group and the transplant group. The high levels of VCAM-1 and ICAM-1, especially in the HD group, have been associated with the role of the kidney in the destruction of these molecules. This condition was explained by the fact that there was no correlation between serum creatinine levels and VCAM-1 in predialysis CRF patients and the transplant group, while a strong correlation was observed between VCAM-1 level and serum creatinine in the HD group.24
In a study by Papayianni et al., it was stated that higher levels of adhesion molecules in HD patients may be due to the pro-inflammatory cytokine increase induced by the uremic environment.25 In our study, high levels of VCAM-1, ICAM-1 and IL-8 in both predialysis CRF and HD groups suggest that they may play a role in endothelial activation, inflammatory process and atherosclerosis by increasing the level of these molecules in the uremic environment.
Decoy Receptor 3 protein is a pleiotropic molecule thought to play a role in inflammation, autoimmunity and cancerogenesis.11 Both tissue expression and circulating levels of DcR3 have been investigated in different types of cancer, and it has been shown that DcR3 levels are increased especially in solid organ tumors.26 It has been stated that in addition to its antiapoptotic properties DcR3 plays a role in immunomodulation. It has been shown in different studies that it plays a role in the maturation of dendritic cells and facilitates leukocyte adhesion by increasing the expression of ICAM-1, VCAM-1 and IL-8.27,28
Bamias et al., showed that DcR3 facilitates the progression to chronic inflammation by inhibiting the apoptosis of inflammatory cells.29 In their study on hemodialysis patients, Hung et al. divided the patients into 3 categories according to their serum DcR3 levels and the risk of cardiovascular mortality was found to be higher in the group with increased DcR3 levels, so DcR3 could be an independent risk factor in cardiovascular risk assessment in the long-term.29 In the same study, VCAM-1 and ICAM-1 levels were also found to be higher in HD patients. It has also been stated that IL-6 levels, which play a key role in the pathogenesis of inflammation and atherosclerosis, increase in correlation with DcR3.30 There are different studies in the literature on the relationship between DcR3 and atherosclerosis. In the study of Chang et al. the severity of the coronary artery disease (CAD was determined by the Syntax score, and it was stated that DcR3 was an independent predictor for a high Syntax score.31 Similarly, in another study conducted with CAD patients who underwent CABG, DcR3 levels were found to be higher than the control group and it was stated that DcR3 is an independent risk factor for CAD (OR=1.8 (95% CI 1.3–2.6)).32 However, in the study conducted by Chen et al., plasma DcR3 levels were found to be lower in the group with severe coronary artery disease compared to individuals with mild and moderate CAD, according to the classification made based on the American Heart Association (AHA) recommendations.33 As is seen, there are conflicting data in the literature. In our study, the positive correlations between DcR3 and CIMT in the dialysis group and between DcR3 and ICAM-1 in the pre-dialysis CRF group tend to support the relationship of DcR3 with inflammation and atherosclerosis.
Similar to other inflammatory markers in our study, serum DcR3 levels of predialysis CRF and HD patients were found to be significantly higher than the transplant and the control groups. Levels in the transplant group were also significantly higher than in the control group. The high level of DcR3 in HD and predialysis-CRF groups, as in other inflammatory serum markers, suggests that the elevation of DcR3 may be related to uremia. As mentioned earlier, DcR3 is known to increase the expressions of VCAM-1, ICAM-1 and IL-8. From this point of view, uremia-triggered endothelial dysfunction and DcR3 may be responsible for the elevation of VCAM-1, ICAM-1 and IL-8.
In a study conducted by Turkmen et al., ischemia modified albumin (IMA) levels of the renal transplantation group were shown to be higher than the control group.4 IMA is a biomarker associated with oxidative stress and inflammation.34 The fact that DcR3, together with other serum markers evaluated in our study, was found to be higher in the transplanted group compared to the control group supports the hypothesis that the inflammatory process does not recover completely even though transplantation is performed.
CIMT is one of the simple and inexpensive atherosclerosis risk indicators.35 It has an important role in the evaluation of subclinical atherosclerosis, prediction of coronary heart diseases and vascular events. Its noninvasive measurement is also advantageous.36 It is known that cardiovascular diseases take the first place among the causes of mortality in patients with CRF and the prediction of subclinical atherosclerosis becomes even more important for CRF patients. It has been stated that the presence of carotid artery plaque is more valuable than CIMT in the prediction of coronary artery diseases. It has been indicated that CIMT may increase depending on age, but the presence of plaque is more frequently associated with inflammation, oxidative stress, endothelial dysfunction, and smooth muscle cell proliferation, so it will contribute more than CIMT to the prediction of many cardiovascular diseases including myocardial infarction.37
In our study, CIMT was lower in the transplant group compared to predialysis CRF and HD groups and higher than control. When the results of other biomarkers in our study are evaluated in line with the literature data, it can be said that renal transplantation contributes to the reduction of the inflammatory process, but does not completely reverse inflammation.
Considering the limitations of the study, living donor kidney transplantation is common in Turkey, and generally young people can find transplantation opportunities. Therefore, there is a significant difference in the mean age between the pre-dialysis CRF and transplant groups. Another limitation is that the study is cross-sectional. It is thought that prospective studies are needed to evaluate the role of DcR3 in cardiovascular risk assessment in detail. Nevertheless, this cross-sectional study supports the DcR3-cardiovascular risk association.
The differences between the groups in terms of age, the limited number of samples, its cross-sectional design and the fact that it was performed in a single center are the important limitations of the study. Kidney transplantations from living donors are more common than cadaveric renal transplantations in Turkey. Generally, young patients can find the opportunity to transplant. Therefore, there is an age difference between the transplanted group and other patient groups. Comprehensive studies are needed to further address the role of DcR3 in cardiovascular risk assessment. Nevertheless, this cross-sectional study is thought to contribute to the elucidation of the relationship between DcR3 and cardiovascular risk.
ConclusionsIn conclusion, the high level of DcR3 together with other inflammatory markers and CIMT in the uremic environment suggests that DcR3 plays a role in the progression to inflammation and atherosclerosis. Considering its pleiotropic effects, it is difficult to say whether the elevation of DcR3 is the cause or effect of inflammation or it increases as a compensatory response. In this respect, more comprehensive studies are needed to support its relationship with uremia using pathway analyzes.
Conflict of interestNone.