Introducción y objetivos: El hiperparatiroidismo secundario es una complicación habitual en pacientes con insuficiencia renal crónica. El tratamiento con paricalcitiol, activador selectivo del receptor de vitamina D, ha demostrado tener beneficios en el tratamiento de estos pacientes al disminuir adecuadamente la hormona paratiroidea (PTH) con mínimas variaciones del calcio y fósforo séricos. El objetivo de este estudio es evaluar la efectividad y la seguridad del paricalcitol en el tratamiento de pacientes con insuficiencia renal crónica (ERC 3 y 4). Métodos: Se llevó a cabo un análisis de datos de nuestra experiencia, en condiciones de práctica clínica habitual, en 92 pacientes de más de 18 años con diagnóstico de ERC de grado 3 y 4. Los pacientes incluidos en el mismo fueron tratados con paricalcitol y evaluados mediante controles periódicos cada tres meses. Como medida principal de efectividad se estableció la obtención de dos disminuciones en visitas consecutivas ≥del 30% de la hormona paratiroidea intacta (PTHi) respecto a las cifras basales. Se analizaron como objetivos secundarios el cumplimiento de los objetivos de acuerdo con las guías de la Sociedad Española de Nefrología (S.E.N.) y Kidney Diseases Outcome Quality Initiatives (K/DOQI), y, también, la relación entre la efectividad del tratamiento y las diferentes variables registradas de los pacientes. La variable principal de seguridad estudiada fue la aparición de hipercalcemia. Resultados: El objetivo principal del estudio lo cumplieron el 54,3% de los pacientes. Adicionalmente, en otro 16,3% de los pacientes disminuyó la PTHi más del 30% al llegar a la tercera visita (a los seis meses). En conjunto, un 70,6% de los pacientes habían conseguido reducir más del 30% los niveles de PTHi a los seis meses con el tratamiento con paricalcitol. La relación entre el éxito del tratamiento y el grado de filtrado glomerular fue significativa, así como su relación con el índice de masa corporal. Apenas hubo efectos adversos; se halló hipercalcemia en un 2,1% de los pacientes. Conclusiones: El tratamiento con paricalcitol presenta una buena efectividad en el control del hiperparatiroidismo secundario en pacientes no en diálisis, bajo condiciones de práctica clínica habitual con un alto grado de seguridad.
Purpose: Secondary hyperparathyroidism is a common complication in patients with chronic kidney disease. Treatment with paricalcitol, a selective vitamin D receptor (VDR) activator, has shown benefits in these patients by adequately reducing PTH levels with minimal changes in serum calcium and phosphorus. The aim of this study was to assess the effectiveness and safety of paricalcitol in chronic renal disease patients (CKD grades 3 and 4). Methods: A study of our experience with paricalcitol was conducted in normal clinical practice in patients over 18 years diagnosed with grade 3 or 4 chronic kidney disease. Patients were periodically evaluated every 3 months. The primary endpoint of effectiveness was to obtain two consecutive decreases of ≥30% in iPTH with respect to baseline values. The secondary endpoints were fulfilment of the objectives in accordance with the Spanish Society of Nephrology (SEN) and Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines, as well as the relationship between the effectiveness of the treatment and different patient variables. Safety was studied by means of hypercalcaemia events. Results: The primary study endpoint was achieved in 54.3% of patients. In addition, another 16.3% of patients had reduced iPTH by more than 30% at the 3rd visit. Therefore, 70.6% of patients reduced their iPTH levels by more than 30% in 6 months. The relationship between treatment success and both glomerular filtration rate and body mass index was significant. There were few adverse events, although hypercalcaemia was found in 5.4% of patients. Conclusions: Treatment with paricalcitol is effective in controlling secondary hyperparathyroidism in non-dialysed patients with a wide safety margin.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) is a frequent and early complication of patients with chronic kidney disease (CKD), characterised by increased levels of parathyroid hormone (PTH). It is usually accompanied by hyperplasia of the parathyroid glands as well as high morbidity.1,2
Metabolic changes of this disorder are caused by a progressive loss of renal mass and a decline in the glomerular filtration rate (GFR). During progressive renal failure, PTH increases in inverse proportion to the decrease in GFR.3
The pathogenesis of SHPT has long been attributed to 3 main factors: calcitriol deficiency, hyperphosphataemia and hypocalcaemia.
According to some observations, in early stages of CKD (GFR >70 ml/min), an increase of phosphoraemia, detected only after phosphorus overload following ingestion, explains the early onset of SHPT.4 Moreover, the decrease in calcium and occasional increases in phosphorus have an undeniable role in stimulating the parathyroid glands. However, it is regularly seen in clinical practice that the levels of serum calcium and phosphorus are within normal range until the relatively advanced stages of CKD.3-6
Furthermore, a new pathogenic factor, FGF23 (Fibroblast Growth Factor 23) has been discovered in recent years. Although not many details are known about its mechanism of action, this phosphatonin, synthesised primarily in the bone, helps to prevent a hypothetical phosphorus overload via its phosphaturic action while decreasing the synthesis of calcitriol.7,8
SHPT and mineral metabolism disorders have 2 main types of clinical consequences: the first in the musculoskeletal system and the second in the cardiovascular system. The first is due to increased bone remodelling, whose usual injury is fibrous osteitis, with a loss of bone mass and structural integrity of the bones.9,10 These renal osteodystrophy lesions have been documented via bone biopsies in very early stages of CKD.11,12 The second is associated with an increased risk of cardiovascular calcification, with vascular toxicity playing an important part.9,10,13,14
The usual treatment for SHPT includes restriction of dietary phosphorus, phosphorus binders and administration of vitamin D receptor activators. More recently, calcimimetic cinacalcet has been added to the therapeutic arsenal. This activates the calcium receptor and inhibits PTH secretion, in addition to having other effects.
Calcitriol is the most frequently used vitamin D analogue. However, available data shows it to have the significant disadvantage of increasing the absorption of calcium and phosphorus, with the consequent risk of hypercalcaemia. This may lead to increased vascular calcification and an increased cardiovascular mortality risk in certain circumstances.15
Therefore, new drugs to activate the vitamin D receptor have been developed with less effect on the intestinal absorption of calcium and phosphorus. Paricalcitol is a third-generation drug that selectively activates the vitamin D receptor (VDR), depending on the tissue.16
It has been shown experimentally to suppress PTH secretion, but with minimal changes in calcaemia and phosphoraemia.17-19 Moreover, there is evidence in animal models showing that treatment with paricalcitol does not increase the expression of procalcifying markers in vascular smooth muscle cells. This does not happen with vitamin D analogues, which do overexpress some of these markers, increasing vascular calcification. This vascular calcification protective effect, typical of paricalcitol, is independent of serum calcium, phosphorus or PTH.20,21
Several clinical trials and post-authorisation studies have shown that paricalcitol is able to control the effects of SHPT with less hypercalcaemia and hyperphosphataemia than calcitriol in haemodialysis patients.14-26 One well-known study observed an increase in survival after a 2-year follow-up for haemodialysis patients who switched to paricalcitol from calcitriol.23
Another reason for this study was the current lack of clinical experience of non-dialysis patients with early hyperparathyroidism treated with paricalcitol.
PATIENTS AND METHODS
Patient selection
Those included were stable patients over 18 years of age, without concomitant severe disease, with an established grade 3 or 4 diagnosis of CKD. They were referred for the first time to the Nephrology Department of the San Cecilio Clinical Hospital, Granada from primary care or another consultation in our hospital between October 2008 and June 2010.
The inclusion criteria were to present SHPT with intact parathyroid hormone (iPTH) values >70 pg/ml for patients with CKD grade 3 and iPTH >110 pg/ml in patients with CKD grade 4. Patients were excluded if they had total serum calcium>9.5 mg/dl or had received treatment with vitamin D, vitamin D analogues, phosphate binders, calcium salts, drugs that might alter calcium or bone metabolism such as bisphosphonates and/or calcitonin in the previous 6 months. Patients with significant comorbidity or an inability to continue treatment were also excluded.
Objectives and endpoints
The objective was to establish the effectiveness and safety of oral paricalcitol administration in patients with CKD grades 3 and 4 (estimated MDRD GFR of 59-30ml/min/1.73m² and 29-15ml/min/1.73m², respectively) under the usual clinical practice conditions at our hospital. We retrospectively analysed our clinical experience by considering the main analysis variable as the effectiveness of oral paricalcitol, as described previously, in achieving 2 consecutive reductions of 30% or more from baseline iPTH.
The secondary effectiveness variable analysed was the percentage of patients who fulfilled the Spanish Society of Nephrology (SEN) and the Kidney Foundation Disease Outcomes Quality Initiative (K/DOQI) guidelines. These included iPTH <70pg/ml (CRD 3) and iPTH <110pg/ml (CRD 4), with calcium in the range 8.4-9.5mg/dl and phosphorus in the range 2.7-4.6mg/dl.
In addition, the relationship between treatment success and different patient variables were analysed.
The primary safety variable in the study was the follow-up of total calcium values to detect the presence of hypercalcaemia with a total calcium >10.5mg/dl.
Follow-up and medication procedures
The treatment follow-up was retrospective analysis at 6 months by comparing data from the baseline visit (V1) with two successive visits with a 3-month interval, V2 (second visit) and V3 (third visit). The diagnosis, prescription and follow-up were carried out by a single doctor.
The initial dose of paricalcitol was determined according to the baseline concentrations of iPTH described in another study.22 A daily dosing regimen was chosen, with an initial dose of 1µg per day when the iPTH was ≤500pg/ml, and 2µg per day when ≥500pg/ml.22
At the following quarterly visits, the dose was modified according to the iPTH response following the usual method in our clinic. If the reduction of iPTH was <15%, the dose was doubled; if the reduction was between 30% and 60%, it was maintained; and if the reduction was >60%, it was halved. If serum calcium was >10.2mg/dl, the dose was reduced, and if it was >10.5mg/dl, the medication was temporarily suspended. Fortnightly determinations of calcium were then performed and the therapy restarted after normalisation of serum calcium.
Patient clinical data from the central computerised and centralised hospital medical records was taken and analysed statistically. They included the age, sex, body mass index (BMI), CKD etiology, concomitant treatments, serum calcium level, serum phosphorus, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, haemoglobin, calculated creatinine clearance, Cockroft-Gault GFR, estimated MDRD GFR and calcidiol levels (25-OH vitamin D). Patients were asked beforehand for permission to use these data for the computer analysis via a data confidentiality agreement.
Laboratory methods
Total calcium and phosphorus serum levels, as well as the other analytical parameters, were measured with an autoanalyser according to hospital laboratory procedures. The calcium values in this study were expressed as uncorrected total calcium levels with serum protein or albumin levels. PTH was determined by electrochemiluminescence (EQL Elecsys PTH, Roche), and the results corrected with the coefficient 0.97 to express them as IRMA NICHOLS according to the K/DOQI guidelines.
Statistical analysis
A descriptive analysis of the categorical variables was conducted by calculating the frequency of each. For quantitative variables, the mean and standard deviation were calculated when the distribution was normal; and the median and interquartile range when the distribution was not normal. Some quantitative variables were categorised.
Statistical significance was assessed using the chi-square test (for categorical variables), Student's t test or the nonparametric Wilcoxon test as appropriate (for quantitative variables). The univariate association analysis was performed using the chi-square test, Student's t-test or the Mann-Whitney U test.
The relationship between treatment success and different variables was analysed in a univariate or multivariate logistic regression. The goodness of fit was assessed by the Hosmer and Lemeshow test.24
Calcium and phosphorus levels were measured to evaluate the safety of the treatment by analysing the appearance of hypercalcaemia or hyperphosphataemia. Calcaemia was categorised into four levels as follows: ≤8.5mg/dl, >8.5 and ≤9.5mg/dl, >9.5 and ≤10.5mg/dl and >10.5mg/dl. The distribution of patients in each category was studied. Also, the presentation of moderate hypercalcaemia was analysed (percentage of patients with a value >10.2mg/dL in at least one visit), which resulted in the dose having to be reduced, and significant hypercalcaemia (percentage of patients with a value >10.5mg/dl) that forced the temporary suspension of treatment.
RESULTS
Demographic and baseline parameters
Initially, 99 patients who met the inclusion criteria were selected, 7 of whom had incomplete data at 6 months (1 for voluntarily discontinuing treatment and 6 because the follow-up was lost before the 3rd visit). Therefore, the final analysis was of 92 patients who had completed 6 months treatment.
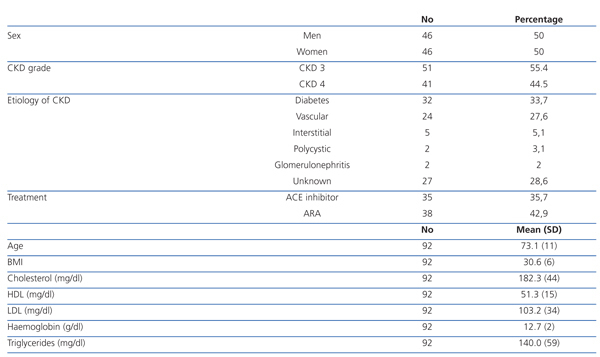
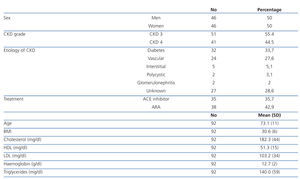
The mean age was 73±11 years, and 50% were women. There was a higher frequency of moderate CKD (55.4% with CKD 3) than serious CKD (44.5% with CKD 4). The most frequent etiology of CKD was diabetes (33.7%), followed by vascular (27.6%), interstitial (5.1%) and polycystic kidney disease (3.1%). There were 28.6% of cases with unknown etiology. The mean body mass index (BMI) was 30.6±6, indicating an overweight population. The mean total cholesterol was 182.3±44mg/dl, HDL cholesterol was 51.3±15mg/dl and LDL cholesterol was 103.2±33mg/dL, which meant a relatively controlled lipid profile in most patients. There were 35.7% of patients under treatment with angiotensin-converting enzyme (ACE) inhibitors and 42.9% with angiotensin II receptor antagonists (ARBs) for associated hypertension (HT), see Table 1.
Analysis of treatment effectiveness
The planned review sequence was quarterly, with the average actual time being 11.7±3 weeks for the second visit (V2) and 25.6±6 weeks for the third (V3).
The mean paricalcitol doses administered at the 3 study times were as follows: at the baseline visit, patients were prescribed 7.4±2.4mg/week, at the 2nd visit it was 6.8±2.4mg/week and in the 3rd visit it was 5.2±3.2mg/week.
The primary outcome of the study was achieved in 54.3% of patients, who had a reduction of ≥30% in iPTH levels at the 2nd visit (V2, 3 months) which was maintained at the 3rd visit (V3, 6 months). In addition, another 16.3% of patients achieved a decrease of ≥30% in iPTH levels at the 3rd visit (V3). Thus, 70.6% of all patients had decreased their iPTH by over 30% at 6 months, compared to baseline.
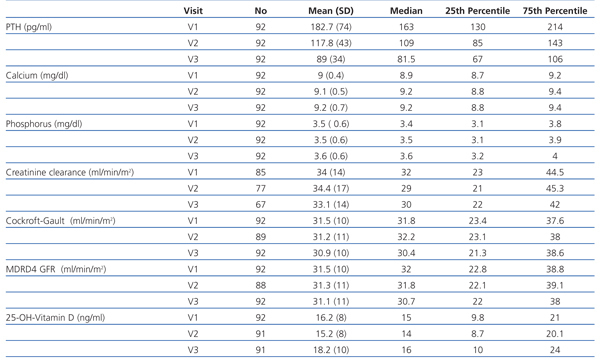
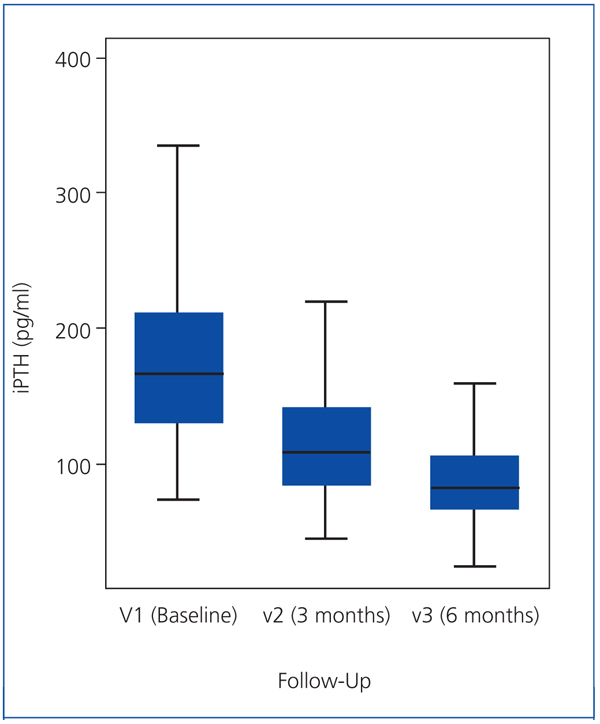
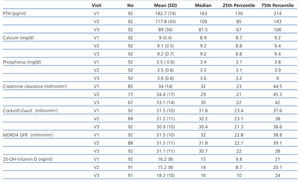
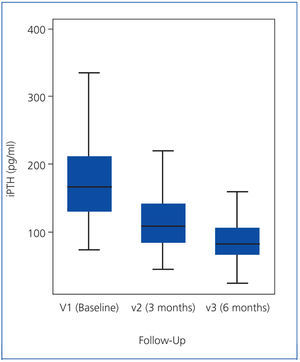
As the iPTH values did not follow a normal distribution, they were expressed as median and interquartile range (Figure 1). The median iPTH shows a statistically significant decrease of 33.1% between baseline (V1) and the 2nd visit (V2), from 163 to 109pg/ml (P=.001). There was also a statistically significant change between the 2nd (V2) and 3rd (V3) visits: decreasing by 25.2% from 109 to 81.5pg/ml (P=.001). Overall, for all patients, over 6 months of the study, the median iPTH decreased significantly by 50.0%, from 163 to 81.5pg/ml (P=.001), see Table 2 and Figure 1.
The reductions in iPTH allowed a proportionately lower dose of paricalcitol to be used, from 7.4±2.4 to 6.8±3.08µg/week (V2) and 5.2±3.2µg/week (V3).
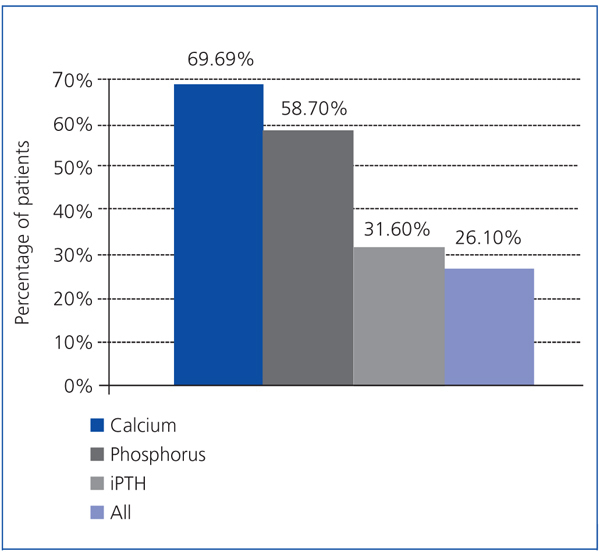
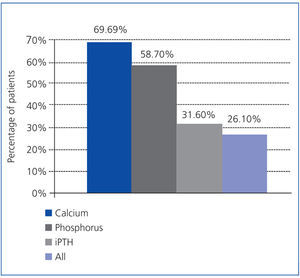
As a secondary objective, the treatment effectiveness was analysed according to the K/DOQI guidelines, which took into account the calcium and phosphorus values, in addition to PTH. The proportions of patients meeting these target levels at 6 months were: iPTH 31.6%, calcium 69.6% and phosphorus 58.7%, although only 26.1% of patients achieved all these objectives simultaneously (Figure 2).
Variations in total serum calcium (baseline 9.0±0.5mg/dl and end 9.2±0.7mg/dl) were not statistically significant (P=.12). Similarly, changes in serum phosphorus (baseline 3.5±0.6mg/dl and final 3.6±0.6mg/dl) were not statistically significant (P=.2), see Table 2.
The different renal function calculations also showed no significant changes: diuresis (P=.6), Cockroft-Gault GFR (P=.6) and MDRD GFR (P=.7), see Table 2.
We also observed small variations in the levels of calcidiol, which are likely to reflect the seasonal changes due to the different times of year analysed. Their values increased slightly from 16.2±8ng/ml to 18.2±10ng/ml, which was not statistically significant (P=.13), see Table 2.
Relationship between treatment effectiveness and diabetes and chronic kidney disease grade
There were no significant differences in the primary endpoint for diabetic and non-diabetic patients, with the objective being achieved in 56.2% of diabetics and 53.3% of non-diabetics.
Significant differences were detected in the relationship between effectiveness and CKD grade, as the primary endpoint was achieved in 67% of patients with CKD 4 versus 44% of those with CKD 3 (P=.05).
Other factors associated with effectiveness: univariate and multivariate associations
The univariate logistic regression analysis found a significant positive association between the main efficacy variable (probability of treatment success) and increased renal affectation (CKD 4 vs. CKD 3) [odds ratio (OR) of 2.52; 95% confidence interval (CI) of 1.07-5.97] and higher baseline iPTH levels (OR 1.02; 95% CI: 1.01-1.03). While the probability of treatment success was lower for a higher BMI (OR 0.87; 95% CI: 0.79-0.95), higher creatinine clearance (OR 0.97; 95% CI 0.93-0.99) and increased GFR estimate by Cockroft-Gault (OR 0.95; 95% CI: 0.91-0.99).
In the multivariate logistic regression analysis, baseline iPTH was chosen as an explanatory variable, as it was the most statistically significantly successful parameter for the goal of the study. Also, BMI was included in the multivariate model as an additional variable, and the equation was adjusted for the variables of sex and age. The resulting model showed a good fit. The result was an increase of 2% treatment success for each unit increase in baseline iPTH (OR 1.02; 95% CI: 1.01-1.03), and a decrease of 17% for each unit increase in BMI (OR 0.83; 95% CI: 0.75-0.93). In both cases, the results were independent of the age or sex of patients.
Treatment Safety
Most patients had serum calcium levels between 8.5 and 9.5mg/dl during follow-up: 72.4% at the 2nd visit (V2) and 69.6% at the 3rd visit (V3). Only 2 patients (2.1%) had calcium levels above 10.5mg/dl. Moreover, 5 patients (5.4%) were observed with calcium levels above 10.2mg/dl (1 case at V2 and 4 cases at V3).
Also, calcium values below 8.5mg/dl were observed in 9.2% of patients at V2 and 8.7% of patients at V3.
Variations in the mean calcium and phosphorus during the study were not statistically significant.
Other adverse effects
Seemingly transient symptoms of nausea and/or vomiting attributed to the drug were found in 3 patients (3.06%), who were receiving numerous medications. However, because of their transitory nature, it was not necessary to discontinue the medication.
One patient (1.02%), also being administered many drugs simultaneously, dropped out of treatment at the 3rd visit voluntarily.
DISCUSSION
The data collected in this study from our consulting practice, and those applied retrospectively to patients with similar criteria in the literature, show a high effectiveness for paricalcitol in controlling SHPT in patients with CKD stages 3 and 4 when administered in a daily regimen, with no significant changes in calcaemia or phosphoraemia.
There was a significant decrease of 50.0% (P=.001) in median iPTH in our patients from baseline (163pg/ml) to the end of the study (81.5 pg/ml). This significant reduction fulfilled the main objective of the study for 54.3% of patients who achieved ≥30% reductions in iPTH levels at 3 months and maintained this decrease at 6 months; ie, they had 2 consecutive decreases compared to baseline. Our results are smaller in magnitude than those in Coyne's study, where 90% of patients achieved a similar objective.22 We believe these differences are due to the different study conditions. Coyne's study was a controlled clinical trial with a prospectively determined primary objective, while ours was limited by the conditions in a normal hospital clinic and the data were studied retrospectively. Furthermore, Coyne's study was based on PTH levels greater than ours, therefore patients received mean doses of paricalcitol larger than ours. Finally, the time intervals and follow-up times were also different.
Another aspect observed in our patients was that there was a greater reduction (35.5% vs 24.4%) in iPTH between baseline (V1) and the 2nd visit (V2) than between the 2nd (V2) and 3rd visits (V3). This is consistent with previous studies which have shown decreases in iPTH levels of 30% in the first 2 months with progressively less decreases in the following months.18,22,25 In total, we observed a median decrease of 50.0% in iPTH from baseline levels until the end of study (V1 to V3). This meant that 70.6% of patients reduced their iPTH by more than 30% over 6 months.
This reduction compares favourably with similar studies. In a long-term open multicentre study for oral paricalcitol, a progressive decrease in iPTH was seen up to the 13th month of treatment.25 In other studies, the effects of paricalcitol were compared with calcitriol, a non-selective vitamin D analogue, and significantly larger and faster decreases in iPTH were observed with paricalcitol than with calcitriol.26,27 Another recent study conducted with 40.5% of patients receiving cinacalcet in haemodialysis associated with paricalcitol, experienced a 50% reduction in PTH, which was comparable to our patients who only received paricalcitol.28
Moreover, the significant decreases in iPTH in our patients allowed the paricalcitol dose to be gradually reduced from a weekly average of 7.4g at the start to an average of 5.2µg at the end, with the maximum degree of iPTH control. This is a 29.7% reduction in the dose over the 6 months of the study, as found by other authors. In an open, long-term prospective study, the paricalcitol dose was significantly reduced throughout the study while maintaining suppression of the iPTH.26
Another way to analyse the treatment effects on mineral metabolism disorders is to evaluate compliance with values considered optimal by different clinical practice guidelines, such as the K/DOQI guidelines proposed by the American National Kidney Foundation29 and the SEN guidelines.30 According to both guidelines, 31.6% of our patients were within the range for iPTH, 66.9% were within the range for calcaemia and 58.7% were within the range for phosphoraemia at the end of the study period. Also, 26.1% of patients managed all 3 parameter targets simultaneously. It should be noted that the iPTH level treatment algorithm for our patients was not designed prospectively to achieve the margins proposed by these guidelines.
The difficulty in complying with the K/DOQI criteria has long been known, and has been described as a “painful” or “uphill” battle. They had scarcely been published, when it was found that few patients were meeting them. One of the first studies, in haemodialysis patients, found 20% of patients complying with iPTH levels and only 8% for all 3 parameters simultaneously.31 In the Spanish SEN multicentre study, OSERCE II, which included patients with CKD not on dialysis in 39 centres, it was found that 30% were within the K/DOQI range for PTH, 35.6% for calcium and 76.5% for phosphorus. This study highlighted the difficulty of reducing the PTH, especially when compared with another similar study of 3 years earlier by the same authors, which achieved a similar decrease in PTH.32 Similarly, another study in our group collected data from the Sistema de Información de Coordinación Autonómica de Trasplante Andaluza (Andalusian Transplant Autonomy Coordination Information System, SICATA) for the years 2007, 2008 and 2009 concerning the control of PTH according to the K/DOQI guidelines in haemodialysis patients. The percentage within the range for iPTH was 32.4% (in 2007 and 2008) and 35% (in 2009). It should also be noted that between 13.5% and 15% of patients were within the range simultaneously for all 3 parameters: calcium, phosphorus and PTH.33 Finally, a multicentre Italian study (Italian FARO Survey), involving 2,637 patients from 28 haemodialysis centres, also showed the difficulty in complying with the K/DOQI guidelines. There were 26.8% of patients within the iPTH range at baseline and 32% at 18 months.34 Thus, these studies illustrate the difficulty in achieving the guideline objectives for both dialysis and in CKD not on dialysis.
Moreover, as a secondary aim in our study, we evaluated the various factors that could influence the response to treatment. There was no difference between diabetic (56.2% success) and non-diabetic patients (53.3% success) regarding a decrease ≥30% in iPTH levels
However, the treatment was more effective in patients with CKD grade 4 than those with grade 3, for both the primary endpoint and secondary measures (reduction in iPTH in at least 1 visit). In addition, logistic regression results confirmed an association between treatment effectiveness and the CKD grade (greater effectiveness in CKD grade 4, lower creatinine clearance, lower Cockroft estimate or lower MDRD). One possible explanation is that it is more difficult to lower iPTH to a normal range the closer you are to it, and easier the farther away, at least in early hyperparathyroidism where there is only diffuse hyperplasia of the parathyroid glands and they are sufficiently sensitive to the activation of the vitamin D receptor (VDR).
The association with BMI showed that the higher this was, the lower the likelihood of treatment success. Other authors in a recent paper also noted that a higher BMI had a worse response to oral paricalcitol in renal failure before dialysis, and that obesity is a factor in poor treatment response as was seen in our patients.35
Analysis by a multivariate model showed similar results, ie, that the effectiveness of treatment increased with higher baseline iPTH level and decreased with increasing BMI.
Finally, the most common risks of treating patients with CKD grades 3 or 4 with vitamin D analogues are causing hypercalcaemia, hyperphosphataemia and increased Ca x P product. It is known that selective activation of VDRs, as happens with paricalcitol, lowers intestinal absorption of these ions, significantly decreasing these risks, as seen in the Coyne study.22 It emerged from our study, that treatment with paricalcitol, in addition to its effectiveness, leads to safe management of the drug, as only 5 cases of moderate hypercalcaemia >10.2mg/dl (5.4% of patients) were seen, which represented 2.6% of all analytical results at the 6 months follow-up. Moreover, 2 cases (2.1% of patients) had severe hypercalcaemia >10.5mg/dl which forced the temporary suspension of the drug. As reported in other studies,22 a slight average increase in calcium levels of 0.1-0.2mg/dl was seen, which is not statistically significant. There were no significant changes in levels of phosphorus, nor any determination with hyperphosphataemia. Our data are comparable to those of other studies, where the incidence of adverse effects, hypercalcaemia, hyperphosphataemia and high calcium-phosphorus product was similar in both the paricalcitol group and placebo group patients.14,22,25,26 Given the particular importance that selective activation of VDR is acquiring, in both vascular calcification protection36 and its potential pleiotropic effects,37,38 we believe our experience with this drug could be particularly useful, in terms of effectiveness and safety.
Another problem that must be taken into account with treatments is the global tolerance to these substances. In our study, it was observed that paricalcitol was well tolerated. Adverse effects, which were mainly gastrointestinal, were very few, mild and were resolved in a few weeks without discontinuing the treatment. Only 3 patients (3.06%) were affected; while 1 patient (1.02%), taking plenty of other medications, discontinued treatment by choice due to feeling generally unwell and nauseous without vomiting. None of the analytical tests showed any abnormality to explain the symptoms the patient reported. Similar data are found in other studies.14,22
In conclusion, our data suggest that under the routine clinical practice conditions in our clinic, oral paricalcitol can be used as a treatment of choice in early hyperparathyroidism for patients with CKD stages 3 and 4. There is a sufficient degree of efficiency, safety and tolerance, and clinical results comparable to those of published controlled trials could be expected.
The main effort in this study was to try to apply the skills of rigorous, prospective studies with a control group to normal clinical practice. Therefore, the difficulty in validating our data, due to few previous studies in clinical practice conditions similar to ours, is recognized as a study limitation. A second limitation is the small number of patients treated, and a third is the lack of a longer-term follow-up.
Table 1. Demographic and baseline parameters
Table 2. Changes in key variables in the 3 visits
Figure 1. iPTH values (expressed as median and interquartile range)
Figure 2. Percentage of patients after 6 months treatment meeting the K/DOQI guideline values for calcium, phosphorus and iPTH