To the Editor:
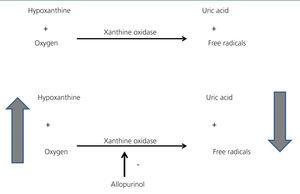
Allopurinol is a xanthine oxidase inhibitor. Xanthine oxidase is an enzyme that has hypoxanthine and oxygen as substrates and uric acid and free radicals as products. The beneficial effects of allopurinol are not only due to the decrease in uric acid, but also the reduction in oxidative stress and the increase in hypoxanthine and tissue oxygen (Figure 1). As such, there are data that support the conclusion that allopurinol improves endothelial dysfunction, decreases vascular oxidative stress, improves myocardial ischaemia and decreases left ventricular hypertrophy.1 Furthermore, several studies have shown that allopurinol reduces total mortality2,3 and in studies on a small number of patients it has been suggested that it reduces the number of cardiovascular events.4,5
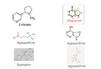
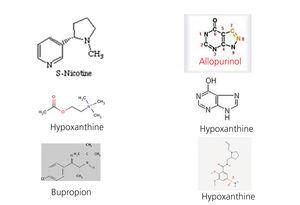
In a previous study on 112 patients with stage 3 chronic renal failure, all without a history of cardiovascular events, we observed that none of the 30 patients who took allopurinol were smokers (0% smokers who received allopurinol compared with 20.73% smokers amongst those who did not receive it; p=.029). As the patient number was very small, we decided to increase the number in order to confirm the results and increase the statistical power. We consecutively questioned 483 patients with diabetes mellitus and kidney disease who came to diabetic nephropathy outpatient clinics for around three consecutive months. We only recorded data on whether they took allopurinol, whether they were active smokers and whether they were ex-smokers. The statistical studies were carried out with SPSS 15.0. In the 483 patients studied, 143 took allopurinol and 340 did not. With regard to the patients who did take it, there was a lower percentage of active smokers (8.3% compared to 15.59%, P=.034) and a higher percentage of ex-smokers (33.56% compared to 13.82%, p=.000) (Table 1). In light of these results, it could be argued that diabetic patients with kidney disease followed up in diabetic nephropathy outpatient clinics and receive allopurinol are less likely to be active smokers and are more likely to be ex-smokers. Given that these results are merely descriptive and do not prove causality, we consider that further studies are necessary in order to obtain more conclusive results. The first question we must consider is whether those who stopped smoking did so because they had higher vascular comorbidity; to determine this, we could use a comorbidity index, but we must remember that in the initial study on 112 patients, none of them had suffered a prior cardiovascular event. The next question to consider would be whether there was any psychological or pharmacological intervention to help them quit smoking. Although all diabetic patients with kidney disease are given psychological assistance to quit smoking, the pharmacological intervention data were not recorded. The next question we must ask is whether there was a temporal relationship between quitting smoking and taking allopurinol, that is, whether the patient quit smoking after beginning treatment with allopurinol. In our study, we recorded the amount of time patients had been taking allopurinol, but it is difficult for the patient to know the exact date on which they stopped smoking. Lastly, it would be necessary to find a mechanism that pharmacologically supports the association found. Figure 2 displays the molecules related to nicotine receptors in the brain. Although we are not experts in pharmacology, we did observe that the nicotine molecule, acetylcholine and bupropion share CH3 groups and that both allopurinol and hypoxanthine molecules are more similar to varenicline molecules with NH groups. Given that there is no xanthine oxidase in the brain, we could consider whether allopurinol would act on other brain enzymes or on receptors in the brain. Nevertheless, given that it has been demonstrated that there is hypoxanthine in the brain, we could suggest that the effect observed is due to an increase in hypoxanthine in the brain secondary to the use of allopurinol, which seems more likely.
In conclusion, patients who take allopurinol smoke less and it is more likely that they are ex-smokers. Given that these findings are merely descriptive, well-designed studies that support this observation would be necessary. While these studies are being implemented, we thought it would be interesting to communicate this to the scientific community, since this finding could be yet another beneficial effect of allopurinol administration.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Percentage of smoker and ex-smoker diabetic patients with kidney disease in accordance with their treatment with allopurinol
Figure 2. Allopurinol and hypoxanthine molecules compared with known molecules that act on nicotine receptors in the brain.
Figure 1. Allopurinol mechanism of action.