En los últimos años se está reconociendo la importancia de las acciones extraesqueléticas de la actividad endocrina de la vitamina D y su profunda interacción con la ERC. Ello ha facilitado el que se pueda disponer de muchos compuestos de vitamina D, tanto nutricional, como activa, con importantes diferencias en su coste económico. En esta revisión se repasa la evidencia disponible sobre la utilidad de los distintos tipos de vitamina D, tanto nutricional como activa, en pacientes con enfermedad renal crónica, tanto en estadios 3-4 como en diálisis, con especial énfasis tanto en su utilidad para el control del hiperparatiroidismo como en su efecto sobre la morbimortalidad. También se analizan los estudios farmacoeconómicos que se han publicado y que comparan entre sí algunos metabolitos activos de la vitamina D. De esta revisión puede concluirse que en el momento actual no existe todavía una base científica suficiente como para preferir la utilización de una vitamina D activa respecto a otra. Mientras no disponga de más datos, el clínico debe seguir las recomendaciones de las guías de práctica clínica, matizadas por la experiencia adquirida con sus pacientes y siempre teniendo en cuenta las implicaciones económicas de sus decisiones terapéuticas.
During recent years, increasing recognition has been given to the endocrine action that vitamin D has on the extraskeletal system, and its deep involvement in CKD. This has meant that many vitamin D compounds (both nutritional and active) have been made available, with an important cost reduction. This review looks at the evidence available regarding the usefulness of different types of vitamin D (nutritional and active) for patients with stage 3-5 CKD and those undergoing dialysis. Emphasis is given to its usefulness to control hyperparathyroidism and its impact on morbidity and mortality. We also analysed pharmacoeconomic studies that have been published which compare active vitamin D metabolites. From this review, we are able to conclude that there is still not enough scientific evidence to be able to prefer one active vitamin D over another. In the meantime, doctors should follow the recommendations given in clinical practice guidelines, always taking into account their personal experience with patients. Furthermore, they must consider the economic impact that their treatment decisions may have.
INTRODUCTION
Constant reference is made to mineral disorders in chronic kidney disease (CKD) patients in clinical practice guidelines, articles and studies, given their importance and their clinical consequences. Vitamin D disorders are main issues related with the responsible pathophysiological mechanisms. A better knowledge of its causes, consequences and possible corrections is important for decision making in daily nephrology practice. Furthermore, understanding the economic cost associated with these decisions can help the sustainability of CKD treatments in Spain. These are the objectives of this article.
PATHOPHYSIOLOGY OF VITAMIN D DEFICIENCY IN CHRONIC KIDNEY DISEASE PATIENTS
Nephrologists could choose between different types of vitamin D. It means they must have a basic understanding of the pathophysiology of vitamin D deficiency in CKD patients. For decades it has been known that a deficiency in 1,25-dihydroxyvitamin D3 (calcitriol or 1,25[OH]2D3, the active metabolite of vitamin D3) is due to a lower activity of renal 1-α-hydroxylase enzyme in CKD patients. Recently, it has also been reported that patients with stage 3-4 CKD,1,2 undergoing haemodialysis3 or peritoneal dialysis4 also suffer from 25-hydroxyvitamin D3 (calcidiol or 25[OH]D) deficiency, which could further contribute to reduced calcitriol levels.
Vitamin D is synthesised in the skin (Figure1a) by photolytic conversion of 7-dehydrocholesterol to provitamin D3, which through thermal isomerisation is converted into vitamin D3, and transported by plasma proteins (Vitamin D Binding Protein [DBP]) to the liver,5 where, with the participation of cytochrome CYP2R1, 25-hydroxylation is produced, giving way to 25(OH)D3 (calcidiol or hydroxycholecalciferol). Calcidiol is stored in the liver, and when necessary it is released into blood, where it circulates bounded to the protein which transports it to the kidney.
The second phase (Figure1b), the bioactivation of vitamin D, takes place in kidney proximal tubule. As such, through the megalin action (low density lipoprotein receptor which facilitates endocytosis), endocytosis of the DBP/25(OH)D3 complex takes place and, due to the 1-α-hydroxylase action, it is hydroxylased in the mitochondria into 1,25(OH)2D3 or calcitriol. Calcitriol is also a powerful vitamin D receptor (VDR) ligand and when it binds to VDRs, megalin expression is re-stimulated. It is, therefore, a feed-forward mechanism for calcitriol production.
1-α-hydroxylase is regulated by calcium, whether directly or via PTH and phosphorus (P), by direct action but via fibroblast growth factor 23 (FGF23), which together with the Klotho gene also have phosphaturic action (Figure1b). The excess of P and vitamin D stimulates FGF23 from the osteocyte, and together with the Klotho gene, they reduce the CYP27b1 gene expression, which codes for 1-α-hydroxylase synthesis and, consequently suppresses the calcitriol synthesis. This causes the intestine to absorb less P, the renal tubule to reabsorb P and the Cyp24 gene expression to increase. This latter gene codes for 24hydroxylase, the enzyme which inactivates calcitriol,6,7 which in turn reduces Klotho expression. Lastly, the Klotho/FGF23 axis, via CD4, has a direct action on the vascular wall and induces endothelial dysfunction.
Calcitriol is not only a hormone that circulates in the organism, regulatin calcium and bone metabolism, but also has a paracrine effects. In other extrarenal tissues, such as the breast, skin, prostate, lymph nodes, colon, pancreas, spinal chord, brain or placenta, there is activity from enzyme 1-α-hydroxylase and there are vitamin D receptors. Calcitriol be produced in these tissues,8 which provokes local autocrine and paracrine effects. Calcitriol/calcidiol’s biological actions and their synthetic analogues are measured by their vitamin D receptor (VDR) (Figure1C). Activated VDR acts together with the retinoic acid receptor, modifying genetic expression by binding to DNA in the cell nucleus. Recently, it has been suggested that a second mechanism of action exists; in fact, the gene transcription pathway is slow and some rapid responses produced following vitamin D administration cannot be explained.9-12 It is possible that a VDR localised in the cell membrane is capable of promoting the activation of a second messenger which determines the immediate cellular effects. Reduced calcitriol levels may reduce VDR activity.
Physiological basics of morbidity and mortality associated with vitamin D deficiency
As has already been stated, vitamin D deficiency affects more than the traditionally-described biological functions,13-16 all of which are related to cardiovascular morbidity and mortality in CKD patients.17
Different studies have associated vitamin D deficiency with albuminuria as a kidney injury marker.18 Vitamin D deficiency has also been related to hypertension, insulin resistance, diabetes and dyslipidemia.19,20 On the other hand vitamin D supplements (ergocalciferol or cholecalciferol) reduce mortality in institutionalised elderly patients.21
This could be because vitamin D administration has a cardioprotective or renoprotective effect, as observed in experimental models: it inhibits the renin-angiotensin system (RAS), has an anti-inflammatory action (systemic and on the renal interstitium) and reduces proteinuria. This beneficial effect should, however, be clarified given that some experimental studies with nephrectomised rats have shown that paricalcitol administration in patients with kidney failure and low calcitriol levels further reduces calcitriol concentration and increases perivascular fibrosis in heart tissue, regardless of calcaemia, phosphataemia or plasma PTH concentration.22
Vitamin D could have beneficial effects, mainly due to its anti-inflammatory and anti-proliferative activity, and its regulatory action in endothelial dysfunction. Calcitriol can modulate the expression of more than 200 genes involved in cell proliferation and differentiation, apoptosis and angiogenesis. Furthermore, VDR is expressed in activated monocytes and macrophages, dendritic cells and T and B cells, and it has been observed that VDR activation has immunosuppressive and immunostimulating effects.9-11
Its ‘non-classical’ actions depend on the association between diseases that are not related to mineral disorders and vitamin D deficiency, including the pathogenesis and progression of hypertension, cardiovascular diseases, type 1 diabetes mellitus, psoriasis, multiple sclerosis, colon cancer, and prostate cancer.9 Furthermore, since it is involved in cell growth regulation, its action could contribute to preventing tumour progression by reducing angiogenesis and increasing cell differentiation and apoptosis of carcinogenic cells.
Several cohort studies show a significant association between 25(OH)D3 deficiency (calcidiol) and cardiovascular events. In the Ludwigshafen Risk and Cardiovascular Health (LURIC) study, Dobnig et al, followed a cohort of 3258 people with an average age of 65 for 7.7 years. They observed that the mortality rate was 22.6% (62.8% due to cardiovascular diseases [CVD]) and that the calcidiol and calcitriol serum concentrations were inversely correlated with cardiovascular mortality, after adjusting for age and for comorbidities.23 Furthermore, CDV and calcidiol serum concentrations were also inversely correlated in the cohort from the classic Framingham offspring study.24
In summary, active vitamin D reduces high PTH plasma concentrations caused by hyperthyroidism secondary to CKD, and this is only one of its mechanisms to slow down the progression of the disease. Other ‘non-classical’ effects of vitamin D that contribute to the cardiovascular protection are the inhibition of renin-angiotensin system, the reduction of systemic, renal or cardiovascular inflammation, and diminishing proteinuria.25,26
However, the exact mechanisms through which vitamin D protects cardiovascular risk are not known, meaning that more studies to determine the efficacy of the different treatments are needed.
DRUGS. TERMINOLOGY AND ACTIONS
Although there is no standardised terminology, we can classify vitamin D types as such27:
Nutritional or preprohormonal vitamin D
Ergocalciferol. Vitamin D2
Cholecalciferol. Vitamin D3
Both are lacking 25-hydroxy (25[OH]). This vitamin is ‘nutritional’ because it can be replaced by diets rich in vitamin D or oral vitamin D supplements.
Calcidiol or calcifediol (25-hydroxyvitamin D; 25-hydroxycholecalciferol; 25[OH]D3)
This is the form that is most abundant in the body, and it is measured by laboratory tests given that its levels are 1000 times higher than calcitriol, and it has a higher half life than calcitriol (2 or 3 weeks as opposed to 4-6 hours). Although there is no definite consensus, vitamin D ‘insufficiency’ is traditionally defined as 25(OH)D3 levels below 30ng/ml and ‘deficiency’ as serum concentrations below 10-15ng/ml. It is estimated that between 70% to 80% of patients with CKD have a vitamin D deficiency.1,28
Calcitriol (1,25-dihydroxycholecalciferol; 1,25[OH]2D3)
This is the true active vitamin D. Calcitriol is approximately 500-1000 times more active than its precursor 25hydroxycholecalciferol.
Vitamin D receptor activators
Alfacalcidol. Vitamin D3 analogue
Doxercalciferol. Vitamin D2 analogue
Falecalcitriol. Vitamin D3 analogue
Paricalcitol. Vitamin D2 analogue
Maxicalcitol. Vitamin D3 analogue
The last two activators are known as ‘selective’ activators, indicating a greater effect on the parathyroids’ VDR than on the intestine and bones, and a lower risk of hypercalcaemia is associated with them.29
EFFICACY OF THE PHARMACOLOGICAL INTERVENTION WITH VITAMIN D IN CHRONIC KIDNEY DISEASE PATIENTS
Recommendations from the Spanish Society of Nephrology guidelines-Kidney Disease: Improving Global Outcomes.
Many observational and controlled studies have been conducted with vitamin D during the different stages of CKD. This is because of the high prevalence of both nutritional and active vitamin D deficiency in CKD patients, and because it has been recognised how important the physiopathology of bone and mineral disorders in CKD is.
There is growing interest in using these compounds, given vitamin D’s extraskeletal actions and the relationship that vitamin D deficiency has with vascular risk factors and a greater mortality rate. However, there is confusion as to what type of vitamin D should be used for CKD patients (active, nutritional or both), and, in the case of active vitamin D, which form should be used. The pressure from the pharmaceutical industry that markets active vitamin D contributes to this confusion.
Clinical practice guidelines, in particular the Kidney Disease: Improving Global Incomes (KDIGO) Guideline26 and the recently published Recommendations from the Spanish Society of Nephrology (S.E.N.)30 advise:
For stage 1-3 CKD patients:
Measure plasma concentration of 25(OH)D3 (calcidiol) and repeat in accordance with the baseline values and the therapeutic interventions (quality of evidence: 2C of GRADE score31).
Correct the vitamin D deficiency with therapeutic strategies recommended for the general public. The S.E.N. Recommendations suggest administering vitamin D at a dose of 200-800IU/day or a single dose of 16 000IU of calcidiol every 15 or 30 days.
For patients with CKD stage 3-5 not on dialysis:
Correct the vitamin D deficiency with nutritional vitamin D (quality of evidence not defined).
If the plasma PTH concentrations are persistently over the normal values, treatment with active vitamin D preparations is recommended (quality of evidence: 2C).
For patients with CKD undergoing dialysis and persistently high PTH:
1. Treatment with active vitamin D preparations, calcimimetics or a combination of both is recommended to reduce the plasma PTH concentrations (quality of evidence: 2B).
Vitamin D’s studies on morbidity and mortality of CKD patients
However, the evidence available on the impact that administering vitamin D has on CKD patient survival is not very consistent and is from observational studies. Most of the controlled studies do not assess survival as their primary objective, but assess changes to biochemical parameters in the short- and mid-term. Current evidence shows no clear preference for one type of vitamin D over another.
During recent years several systematic reviews and meta-analyses have been published that analyse the usefulness of these drugs in CKD patients. The results from these reviews are summarised below.
Nutritional vitamin D and biochemical changes in CKD patients
A recent meta-analysis32 of 17 observational and 5 controlled studies on the usefulness of nutritional vitamin D (ergocalciferol or cholecalciferol) in CKD patients showed an average reduction of plasma PTH concentrations of 41.7pg/ml in patients from observational studies, and 31.7pg/ml from controlled studies.
However, it demonstrated that among patients treated with nutritional vitamin D, hypercalcaemia cases were more frequent in controlled studies (2% in observational studies and 3% in controlled studies). Hyperphosphataemia was also more frequent (0.8% in observational studies and 7% for controlled studies). Plasma calcitriol concentrations increased in patients treated with vitamin D, according to observational studies.
Active vitamin D and biochemical changes in CKD patients
Two Cochrane reviews, both by the same authors, published in 2009 are the most importan studies. They analyse the influence that nutritional and active vitamin D have on biochemical parameters. The first review included 16 controlled studies in CKD patients that did not need dialysis33; the second included 60 controlled studies on dialysis patients.34
According to the analysed studies in the Cochrane reviews, active vitamin D treatment for CKD patients not undergoing dialysis reduced plasma PTH concentrations by an average of 49.34pg/ml, and increased the frequency of patients with hypercalcaemia (but not with hyperphosphataemia) .
The data are very heterogeneous for patients undergoing dialysis, although it concluded that vitamin D reduced serum PTH concentrations and increased phosphataemia. The frequency of hypercalcaemia did not reach statistical significance, but the authors consider that it could be clinically relevant.
The data available when the reviews were conducted could not determine whether more recently marketed compounds were better than other traditional active drugs such as alfacalcidol or calcitriol. However, in a very recent controlled study compared intravenous alfacalcidol with paricalcitol in 80 patients undergoing haemodialysis there were no differences between the response regarding neither PTH suppression nor the incidence in hypercalcaemia and hyperphosphataemia. It concluded that the two drugs are equally effective in treating secondary hyperparathyroidism.35
Nutritional vitamin D and mortality in CKD patients
Although it is well documented that vitamin D deficiency is associated with a greater risk of cardiovascular mortality, there is no other randomised and well designed study that analyses the effect that nutritional vitamin D has on CKD patients’ mortality.
A recent meta-analysis36 of observational and controlled studies on the effects of vitamin D supplements (nutritional or active) on all types of subjects (CKD patients and the general public) concluded that, although evidence is limited, high dose or moderate dose vitamin D supplements seems to reduce the cardiovascular risk. However, when the meta-analysis was restricted to comparative studies with nutritional vitamin D in the general population, this difference could no longer be noted.
Another recent Cochrane systematic review37 analysed the impact that different forms of vitamin D has on preventing mortality in adults without CKD. It included 50 controlled studies with more than 94 000 patients (average age 74 years; 79% women) and concluded that treatment with cholecalciferol (vitamin D3) during two years reduced the risk of mortality by 6%. Ergocalciferol, alfacalcidol and calcitriol did not influence mortality and the latter two did present a greater risk for hypercalcaemia.
There are no studies showing that these treatments are capable of reduce the progression of kidney disease or decrease cardiovascular mortality. Nor are there studies that have shown that some active vitamin D molecules are better than others. Therefore, clinical evidence is needed to answer some questions that are very relevant to CKD patients: does morbidity and mortality decrease using nutritional or active vitamin D? Should plasma calcidiol concentrations be normalised before administering active vitamin D? Are there any relevant clinical differences between the different types of active vitamin D?
Active vitamin D and mortality in CKD patients
The observational studies reviewed in a study that compares nutritional and active vitamin D38 suggest that administering any active vitamin D dose or compound improves survival for CKD patients, undergoing dialysis or not. However, the two Cochrane reviews mentioned, did not find any differences in the mortality of patients treated with active vitamin D or with a placebo, at any CKD stage. There were not differences in CKD progression fractures, bone pain or the need for parathyroidectimy.
ECONOMIC IMPACT OF DIFFERENT VITAMIN D MOLECULES
General concepts
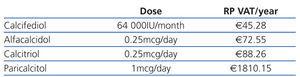
When deciding what to prescribe to a patient, the doctor should bear in mind that the budget is limited and should weigh up the costs and benefits of his/her decision: prescribing drugs with unproven advantages with regards cost-effectiveness ratio is detrimental to the equal distribution of services for other patients. Table 1 and Table 2 show the cost of vitamin D and its analogues in Spain during 2011. The economic differences would not be important if greater costs meant greater effectiveness or usefulness (reduced mortality, reduced frequency in hospitalisation, improved quality of life, etc.).
The results of the cost-effectiveness analysis (CEA) are expressed as the difference in costs divided by the differences in the evolution of morbidity and mortality between two or more strategies.39 The evolution of the disease can be expressed as years of life gained, quality-adjusted life years (QALY) or results on clinical or laboratory aspects. The importance of CEA is mainly to help decide which treatment is more effective and more expensive than its alternative.
As has already been mentioned, incorrectly treating hyperparathyroidism is associated with cardiovascular, bone, and immunological complications, anaemia, etc. The cost of therapeutic options is summarised in Table 1 and Table 2. The decision could be an important burden on the budget, which should not be problematic if the effectiveness of the chosen option was above that of its competitors. Unfortunately, there are no studies that clarify which vitamin D improves survival, and most of the studies’ results are based on comparing the biochemical changes observed in arbitrarily subrogated markers.38
Studies with ergocalciferol or cholecalciferol
As has been previously mentioned, the recent meta-analysis by Kandula et al32 did not contribute with any data on the nutritional vitamin D’s usefulness to reduce cardiovascular and bone problems. Nor is there any evidence that nutritional vitamin D supplements are as effective as active vitamin D or calcimimetics to reduce the hyperparathyroidism in CKD patients.
Clinical practice guidelines recommend nutritional vitamin D supplements for vitamin D insufficiency and deficiency. Although the criteria applied to the general population is not always valid for CKD patients, considering the low cost associated with nutritional vitamin D, it does seem cost-effective to administer it to normalise the vitamin D plasma concentrations.
Studies with selective and non-selective VDR activators
When choosing among the vitamin D receptor activators, we must consider their economic impact, and paricalcitol, a selective activator, has the highest price.
Studies published do not suggest that paricalcitol is more effective than calcitriol to reduce mortality and hospital admissions. There are no randomised prospective studies, and only 2 non-randomised primary studies40,41 and 4 non-randomised supplementary studies have been conducted.42-45 The observational studies have numerous limitations (lack of control of risks, differences in losses to follow-up, differences in severity and in the disease stage). It means that they do not have the same quality of scientific evidence than the randomised studies. Prospective randomised studies must therefore be conducted. There does not seem to be significant differences between intravenous paricalcitol and calcitriol with regards the frequency of hypercalcaemia episodes and/or increased phosphocalcic product.46 Nor were there significant differences between intravenous paricalcitol and alfacalcidol.35 There are no comparative studies between the oral paricalcitol and other vitamin D receptor activators.
Studies that compare paricalcitol’s cost-effectiveness with non-selective activators are inconclusive. A recently published study analysed the cost-effectiveness of paricalcitol compared to alfacalcidol in a hypothetical cohort of patients with stage 3-5 CKD with a decision analysis model using the hidden Markov model.47 Authors concluded that paricalcitol is associated with an increase in cost-effectiveness ratio by $12 840/QALY. However, the study does have many limitations: it assumes that the parenteral formulations are equal to oral ones; data for alfacalcidol are considered equal to calcitriol; there are no data that compare oral paricalcitol with other VDR activators, and the model is only based on observational studies. Furthermore, three of the four authors are employed in the company that markets paricalcitol.
There are also two economic assessments, one American, which compares intravenous paricalcitol and intravenous calcitriol48; and another one is Germany, and compares intravenous paricalcitol with oral calcitriol and intravenous alfacalcidol.49 Both studies conclude that paricalcitol is more cost-effective. The two studies include authors that are employed by the company that markets paricalcitol. Furthermore, the All Wales Medicine Strategy Group (AWMSG) report,50 and the Scottish Medicines Consortium recommendations,51 do not agree with these conclusions as they consider that the analysis’s methodology is not correct, since they are based on non-randomised observational studies.
CONCLUSIONS AND FINAL RECOMMENDATIONS
During recent years, increasing recognition has been given to the endocrine action that vitamin D has on the extraskeletal system, and its rooted involvement in CKD. In consequence many vitamin D compounds (both nutritional and active) have been made available.
However, and as shown in this review, there is still not enough scientific evidence to support the use of one active vitamin D over another. In the meantime, doctors should follow the recommendations given in clinical practice guidelines, always taking into account their personal experience with patients. Furthermore, they must consider the economic impact that their treatment decisions may have.
Based on these premises, the authors of this review recommend:
For patients with 25(OH)D insufficiency or deficiency: administering nutritional vitamin D at the doses recommended by clinical practice guidelines.
For patients with secondary hyperparathyroidism: treating them with active vitamin D in accordance with guideline recommendations, taking into account costs: the most cost-effective drugs would be of first choice. More costly drugs
should be indicated when secondary effects restrict the use of first-choice drugs.
Administering active vitamin D, except when indicated for hyperparathyroidism secondary to CKD, is questionable at present.
KEY CONCEPTS
25(OH)D deficiency is very common in CKD patients and is associated with a greater morbidity and mortality rate.
Although there is no clear evidence on the effectiveness of correcting calcidiol deficiency with nutritional vitamin D to reduce morbidity and mortality, safety and the low cost of these drugs mean that it is recommended for CKD patients with vitamin D insufficiency or deficiency.
Treatment with vitamin D compounds is effective in controlling secondary hyperparathyroidism to CKD. Active vitamin D’s effect is more significant and maintained than that of vitamin D.
There is not enough evidence showing that one type of active vitamin D is better than another, but there is much difference between their costs.
More clinical studies are needed in order to compare different vitamin D compounds with important clinical objectives (mortality, CKD progression, regression of left ventricular hypertrophy).
There are no studies that analyse the benefits of combined nutritional and active vitamin D treatment in CKD patients.
Acknowledgements
The authors would like to thank Dr Santiago Rosales and Dr Victoria Raventós for their invaluable contributions to this manuscript.
Figure 1. Vitamina D Action
Table 1. Cost of oral vitamin D (RP+VAT)
Table 2. Cost of intravenous vitamin D