Chronic kidney disease (CKD) is a common condition that is a major risk factor for cardio-vascular events, end-stage renal disease and all-cause mortality.1 In several large trials, dapagliflozin treatment reduced both primary and secondary renal outcomes at different levels of estimated glomerular filtration rate (eGFR). Dapagliflozin reduced the rate of eGFR decline over time, one of the secondary renal outcomes. 2–5
Glomerular hyperfiltration plays a key role in the progression of CKD, causing intraglomerular hypertension, inflammatory changes, extracellular matrix accumulation and podocyte injury. In addition, loss of eGFR causes compensatory hyperfiltration in the remaining nephrons, leading to further decline in eGFR through glomerulosclerosis and tubulointerstitial fibrosis. Sodium-glucose linked transporter type 2 (SGLT-2) inhibitors are associated with a reduction in glomerular hyperfiltration, probably due to an increase in preglomerular vasoconstriction and a decrease in postglomerular vascular resistance.6
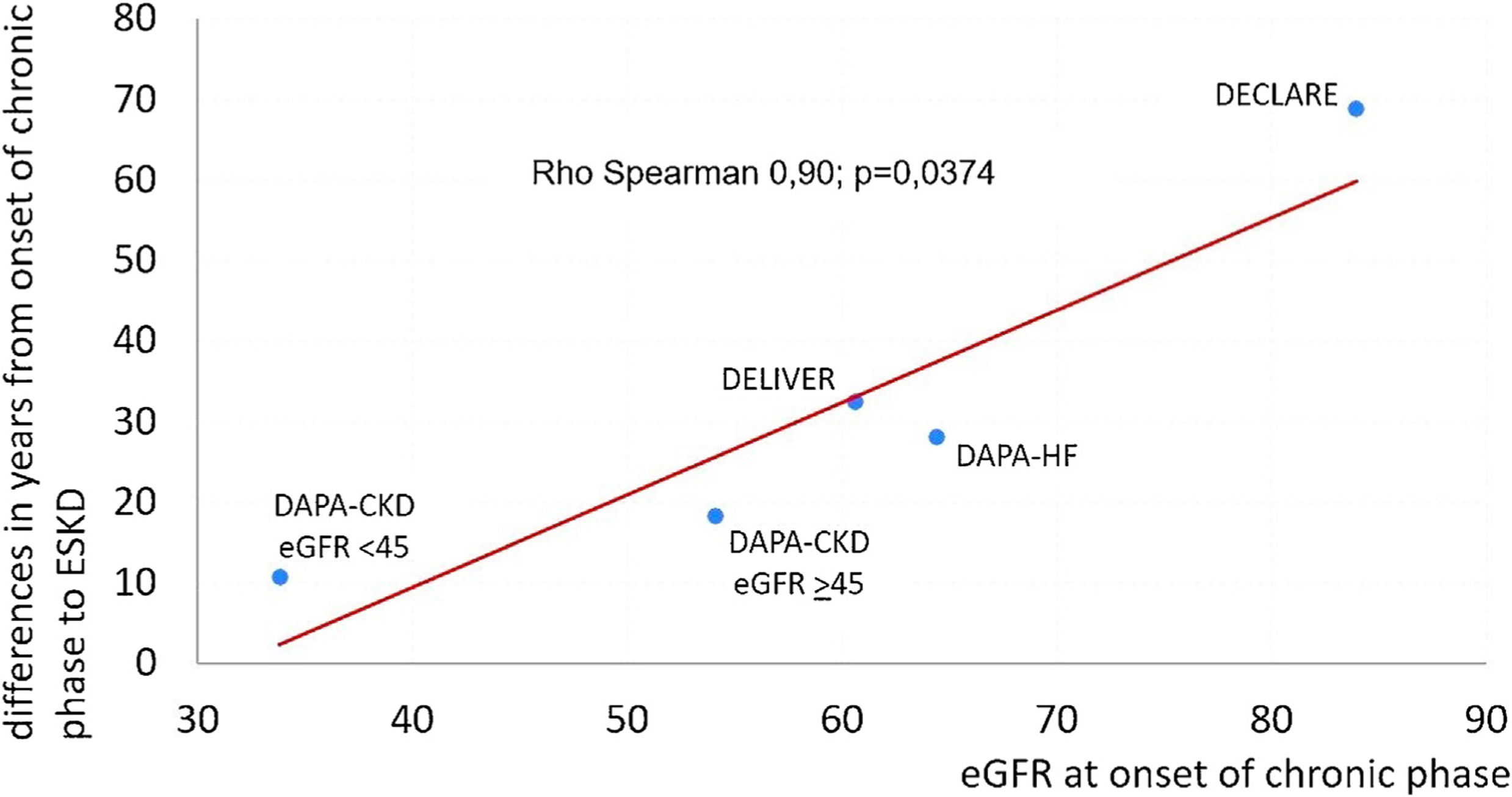
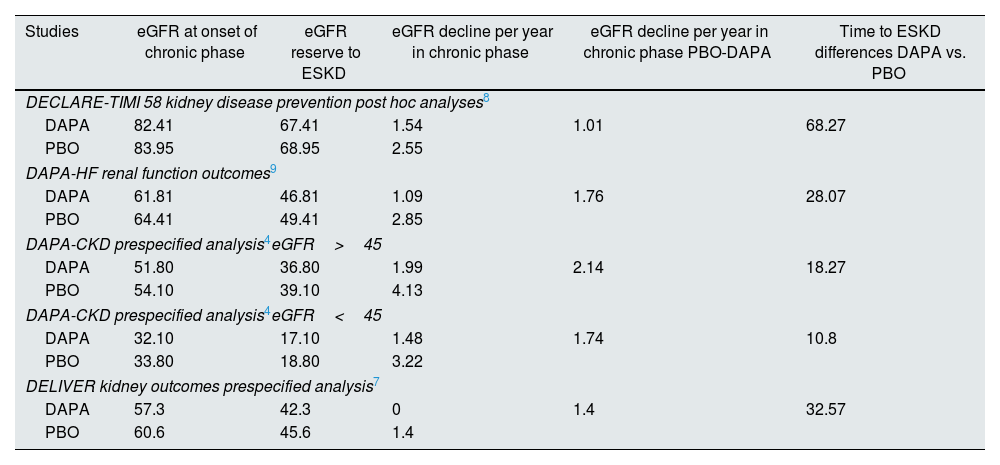
We therefore investigated whether the efficacy of dapagliflozin in slowing the progression of eGFR decline would be influenced by the degree of renal function. A well-known phenomenon observed in studies performed with SGLT-2 inhibitors is a more pronounced fall of the eGFR in treated groups than in PBO groups during the first weeks of treatment (acute phase). This was followed by a partial recovery and thereafter a persistent lower decline of the eGFR until the end of the follow-up period (chronic phase) in treated groups. Thus, for the present analysis we took data from several published studies4,7–9 to evaluate the possible existence of a correlation between eGFR at the onset of chronic phase and differences in time to end stage kidney disease (ESKD). The eGFR reserve to ESKD was calculated as eGFR at onset of chronic phase minus 15. The difference in time to ESKD induced by dapagliflozin intervention was calculated by dividing the eGFR reserve to ESKD in the placebo group by the PBO-DAPA difference in eGFR decline per year during the chronic phase (Table 1). As a result, we found a significant correlation between these two parameters (Rho Spearman 0.90; p=0.0374) (Fig. 1).
Differences between DAPA and PBO groups in time lapse (years) from eGFR at onset of chronic phase to ESKD.
| Studies | eGFR at onset of chronic phase | eGFR reserve to ESKD | eGFR decline per year in chronic phase | eGFR decline per year in chronic phase PBO-DAPA | Time to ESKD differences DAPA vs. PBO |
|---|---|---|---|---|---|
| DECLARE-TIMI 58 kidney disease prevention post hoc analyses8 | |||||
| DAPA | 82.41 | 67.41 | 1.54 | 1.01 | 68.27 |
| PBO | 83.95 | 68.95 | 2.55 | ||
| DAPA-HF renal function outcomes9 | |||||
| DAPA | 61.81 | 46.81 | 1.09 | 1.76 | 28.07 |
| PBO | 64.41 | 49.41 | 2.85 | ||
| DAPA-CKD prespecified analysis4eGFR>45 | |||||
| DAPA | 51.80 | 36.80 | 1.99 | 2.14 | 18.27 |
| PBO | 54.10 | 39.10 | 4.13 | ||
| DAPA-CKD prespecified analysis4eGFR<45 | |||||
| DAPA | 32.10 | 17.10 | 1.48 | 1.74 | 10.8 |
| PBO | 33.80 | 18.80 | 3.22 | ||
| DELIVER kidney outcomes prespecified analysis7 | |||||
| DAPA | 57.3 | 42.3 | 0 | 1.4 | 32.57 |
| PBO | 60.6 | 45.6 | 1.4 | ||
Data taken from referred studies. eGFR: estimated glomerular filtration rate (ml/min/1.73m2); ESKD: end stage kidney disease; DAPA: dapagliflozin; PBO: placebo.
In view of this finding, we believe that it is reasonable to conclude that the nephroprotective effect of dapagliflozin could be related to the degree of renal function. It could be said that from a mechanistic point of view this is not an unexpected finding, since the smaller the mass of functioning nephrons, the fewer the number of points at which the drug can exert its mechanism of action. This may have broad applicability in the clinical setting because although dapagliflozin could delay the disease progression at any stage of CKD, the earlier treatment is established the longer the patient will remain in a less unfavourable clinical condition. Thus, the early initiation of treatment is of particular interest, not only because of its clinical relevance, but also because it can result in substantial cost savings both in terms of reduced pharmaceutical costs and a smaller number of patients on renal replacement therapy.
Conflict of interestAntonio Gippini has received fees for presentations and advisory boards from Amgen, AstraZeneca, Boehringer-Ingelheim, Esteve, Ferrer, Janssen, Lilly, Mundipharma, Mylan, Novartis and NovoNordisk.
Alberto Prado works in Cardiovascular Renal and Metabolism (CVRM) Medical Department of AstraZeneca, Spain.