In recent months, recommendations for the treatment of non-hospitalized patients with mild-moderate COVID-19 and high risk of progression to severe disease included several antiviral drugs (nirmatrelvir/ritonavir, remdesivir and molnupiravir) and monoclonal antibodies (mAB) (primarily sotrovimab in Europe).1 However, there is little documented real-life efficacy in renal transplant (RT) recipients.2,3
We conducted a retrospective cohort study of all RTs with mild-moderate COVID-19 during the period January 1, 2022, to December 31, 2022, who received outpatient treatment in our hospital area. We defined mild-moderate COVID-19 when patients had symptoms related to SARS-CoV-2 infection (diagnosed by PCR and/or antigen) without an indication for hospital admission. We defined severe COVID-19 if patients required hospitalization or died. The indication for drug treatment was made according to known risk factors for disease progression: age > 60 years and/or post-RT time < 2 years and/or comorbidities. The choice of drug depended on anti-S IgG titers (< 1.000 BAU/ml: sotrovimab) and estimated glomerular filtration rate (eGFR) (>30 ml/min/1.73 m2: remdesivir in 3 day regimen; <30 ml/min/1.73 m2: molnupiravir). We did not consider using nirmatrelvir/ritonavir because of the strong drug interactions described with immunosuppressive drugs. Additionally, we collected data from all RT patients requiring hospitalization for COVID-19 during the study period, both treated and untreated prior to admission, as the comparison group.
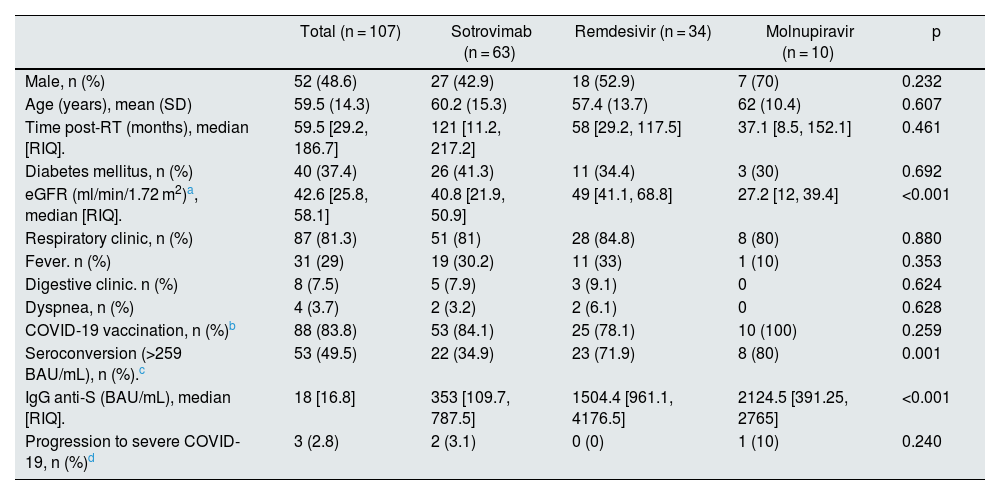
During 2022, 107 RT patients with mild-moderate COVID-19 received outpatient treatment (sotrovimab n = 63, remdesivir n = 34, molnupiravir n = 10) (Table 1). A total of 83.8% were vaccinated at the time of infection according to guidelines provided by Ministry of Health. There were no differences in patient characteristics or clinical manifestations in relation to the drug received, except for the indication criteria for each drug (renal function, anti-S IgG).
Characteristics of patients treated by anti-COVID-19 therapy.
| Total (n = 107) | Sotrovimab (n = 63) | Remdesivir (n = 34) | Molnupiravir (n = 10) | p | |
|---|---|---|---|---|---|
| Male, n (%) | 52 (48.6) | 27 (42.9) | 18 (52.9) | 7 (70) | 0.232 |
| Age (years), mean (SD) | 59.5 (14.3) | 60.2 (15.3) | 57.4 (13.7) | 62 (10.4) | 0.607 |
| Time post-RT (months), median [RIQ]. | 59.5 [29.2, 186.7] | 121 [11.2, 217.2] | 58 [29.2, 117.5] | 37.1 [8.5, 152.1] | 0.461 |
| Diabetes mellitus, n (%) | 40 (37.4) | 26 (41.3) | 11 (34.4) | 3 (30) | 0.692 |
| eGFR (ml/min/1.72 m2)a, median [RIQ]. | 42.6 [25.8, 58.1] | 40.8 [21.9, 50.9] | 49 [41.1, 68.8] | 27.2 [12, 39.4] | <0.001 |
| Respiratory clinic, n (%) | 87 (81.3) | 51 (81) | 28 (84.8) | 8 (80) | 0.880 |
| Fever. n (%) | 31 (29) | 19 (30.2) | 11 (33) | 1 (10) | 0.353 |
| Digestive clinic. n (%) | 8 (7.5) | 5 (7.9) | 3 (9.1) | 0 | 0.624 |
| Dyspnea, n (%) | 4 (3.7) | 2 (3.2) | 2 (6.1) | 0 | 0.628 |
| COVID-19 vaccination, n (%)b | 88 (83.8) | 53 (84.1) | 25 (78.1) | 10 (100) | 0.259 |
| Seroconversion (>259 BAU/mL), n (%).c | 53 (49.5) | 22 (34.9) | 23 (71.9) | 8 (80) | 0.001 |
| IgG anti-S (BAU/mL), median [RIQ]. | 18 [16.8] | 353 [109.7, 787.5] | 1504.4 [961.1, 4176.5] | 2124.5 [391.25, 2765] | <0.001 |
| Progression to severe COVID-19, n (%)d | 3 (2.8) | 2 (3.1) | 0 (0) | 1 (10) | 0.240 |
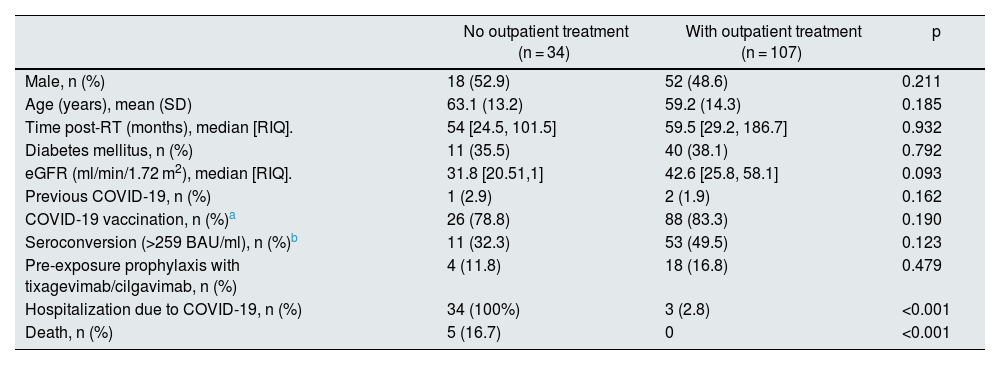
In addition, 37 RT patients were hospitalized throughout the year for COVID-19. Only 3 of them had previously received outpatient treatment (sotrovimab n = 2, molnupiravir n = 1); the rest did not previously contact their RT doctor and already had severe COVID-19 when they attended the hospital, requiring admission. When comparing the recognized risk factors for progression to severe COVID-19, we found no differences between the two groups, treated and not treated, on an outpatient basis (Table 2). Five patients died, all of them in the group that had not received outpatient treatment.
Comparison of characteristics and evolution of patients with outpatient treatment vs. untreated patients.
| No outpatient treatment (n = 34) | With outpatient treatment (n = 107) | p | |
|---|---|---|---|
| Male, n (%) | 18 (52.9) | 52 (48.6) | 0.211 |
| Age (years), mean (SD) | 63.1 (13.2) | 59.2 (14.3) | 0.185 |
| Time post-RT (months), median [RIQ]. | 54 [24.5, 101.5] | 59.5 [29.2, 186.7] | 0.932 |
| Diabetes mellitus, n (%) | 11 (35.5) | 40 (38.1) | 0.792 |
| eGFR (ml/min/1.72 m2), median [RIQ]. | 31.8 [20.51,1] | 42.6 [25.8, 58.1] | 0.093 |
| Previous COVID-19, n (%) | 1 (2.9) | 2 (1.9) | 0.162 |
| COVID-19 vaccination, n (%)a | 26 (78.8) | 88 (83.3) | 0.190 |
| Seroconversion (>259 BAU/ml), n (%)b | 11 (32.3) | 53 (49.5) | 0.123 |
| Pre-exposure prophylaxis with tixagevimab/cilgavimab, n (%) | 4 (11.8) | 18 (16.8) | 0.479 |
| Hospitalization due to COVID-19, n (%) | 34 (100%) | 3 (2.8) | <0.001 |
| Death, n (%) | 5 (16.7) | 0 | <0.001 |
We present the largest series of RT patients with mild-moderate COVID-19 treated on an outpatient basis. Our results suggest that early anti-COVID-19 therapies can halt the progression to severe disease in high-risk patients. Very few of the patients treated as outpatients required admission and none died. Patients admitted without prior treatment had risk factors for the development of severe COVID-19 similar to those in the outpatient group. Considering the favorable evolution of these patients, it is possible that treatment at earlier stages of the disease would also have reduced the need for hospitalization and improved their evolution.
However, loss of efficacy of some of these drugs has recently been reported.1,4,5 Moreover, the incidence of chronic kidney disease (CKD) among RT patients is high, and only molnupiravir and mABs are not contraindicated in patients with eGFR <30 ml/min/1.73 m2.1,6 But recent studies have questioned the efficacy of these two drugs, and both are already deauthorized in many countries.4,5 Nirmatrelvir/ritonavir, in addition to being contraindicated in CKD stages greater than G3b, cause important drug-drug interactions that have limited its use in transplant recipients.7 Finally, remdesivir has shown efficacy in the treatment of mild-moderate COVID-19, as we have also observed in this series.8 Again, this drug is not recommended with eGFR <30 ml/min/1.73 m2. However, initial experiences in patients with reduced eGFR, even on hemodialysis, show adequate efficacy and safety.1,9
Our study has limitations. It is a retrospective cohort, with the limitations inherent to this design. We do not have a control group, although we have analyzed, as a comparison group, all patients admitted for COVID-19 during the study period, who represent the population that developed severe disease among the RT patients in our area. Furthermore, this is a cohort with COVID-19 acquired during the omicron period, so the results cannot be extrapolated to possible new variants.
In conclusion, early treatment of COVID-19 in RT patients seems to reduce the risk of progression to severe COVID-19 and mortality. The lack of efficacy recently observed with some drugs and the contraindication of others raises questions about how to treat high-risk RT patients with CKD. Until new effective and safe therapies become available, we believe that it may be necessary to extend the experience with remdesivir to this group of RT patients with CKD, given the positive clinical results.
FinancingThis research has not received specific support from public sector agencies, commercial sector or non-profit entities.