The information available on the incidence and the characteristics of patients with acute renal failure (ARF) related to drugs is scarce.
ObjectivesTo estimate the incidence of drug-related ARF in hospitalised patients and to compare their characteristics with those of patients with ARF due to other causes.
Material and methodsWe selected a prospective cohort of patients with ARF during hospital admission (July 2010–July 2011). Information on patients’ demographics, medical antecedents, ARF risk factors, ARF severity according to the RIFLE classification and hospital drug administration was collected. We analysed the relationship of drugs with the ARF episodes using Spanish Pharmacovigilance System methods and algorithm.
ResultsA total of 194 cases had an episode of hospital-acquired ARF. The median age of patients was 72 years [IQR 20]; 60% were men. The ARF incidence during hospitalisation was 9.6 per 1000 admissions. According to the RIFLE classification, a risk of kidney damage or kidney injury was present in 77.8% of cases. In 105 (54.1%) cases, ARF was drug-related; the drugs most frequently involved were diuretics, agents acting on the renin–angiotensin system, immunosuppressants, β-blocking agents, calcium channel blockers, contrast media and non-steroid anti-inflammatory drugs. Patients with drug-related ARF had more multi-morbidity, fewer ARF risk factors and lower mortality.
ConclusionsHalf of ARF episodes during hospitalisation were drug related. Patients with drug-related ARF had higher cardiovascular morbidity than those with ARF related to other causes, but they had a lower frequency of ARF risk factors and mortality.
La información sobre la incidencia de insuficiencia renal aguda (IRA) intrahospitalaria relacionada con medicamentos y las características de los pacientes es escasa.
ObjetivoEstimar la incidencia de IRA relacionada con medicamentos en pacientes hospitalizados y comparar sus características con las de los pacientes con IRA relacionada con otras causas.
MétodosCohorte prospectiva de pacientes con IRA intrahospitalaria (julio de 2010-julio de 2011). Se recogió información sobre características y antecedentes de los pacientes, factores de riesgo y gravedad de la IRA según la clasificación RIFLE, y medicación durante la hospitalización. El análisis de la imputabilidad de los fármacos y la evaluación de la relación causal se realizó siguiendo los métodos y el algoritmo del Sistema Español de Farmacovigilancia.
ResultadosUn total de 194 casos presentaron un episodio de IRA intrahospitalaria. La edad mediana de los pacientes fue de 72 años (RI 20); el 60% eran hombres. La incidencia de IRA intrahospitalaria fue de 9,6 por cada 1.000 ingresos. Un 77,8% de los casos presentaron riesgo o daño renal según la clasificación RIFLE. En 105 (54,1%) casos, la IRA se relacionó con medicamentos; principalmente diuréticos, medicamentos que actúan sobre el sistema renina-angiotensina, inmunosupresores, bloqueadores β-adrenérgicos, bloqueantes de los canales de calcio, medios de contraste y antiinflamatorios no esteroideos. La morbilidad cardiovascular fue mayor y la frecuencia de factores de riesgo de IRA y la mortalidad menores en los pacientes con IRA relacionada con medicamentos.
ConclusionesLa mitad de los episodios de IRA intrahospitalaria se relacionaron con medicamentos. Los pacientes con IRA relacionada con medicamentos presentaron más antecedentes patológicos cardiovasculares, pero menos factores de riesgo de IRA y una menor mortalidad.
Acute renal failure (ARF) is a common and severe complication that may develop during hospitalisation and may affect between 5 and 7% of hospitalised patients, with a mortality rate between 20 and 70%, irrespective of treatment.1 The incidence of ARF depends on what definition of AKI was applied in the specific study.2,3
Advanced age, males, infections, history of heart disease, and chronic renal failure (CRF) have been described as some of the ARF-associated risk factors present during hospitalisation.4,5 In addition to mortality, ARF increases the risk of metabolic acidosis, hyperkalemia, arrhthmias, gastrointestinal bleeding, and the risk of neurological abnormalities due to electrolytic imbalance.4 Based on the type of study and the definition of ARF, 11–40% of patients with ARF require dialysis during hospitalisation.4 Moreover, worse renal impairment is associated with prolonged hospitalisation.6
Taking into consideration both community-acquired and nosocomial episodes, drug-related ARF has been described in 18–27% of overall IRA.7 Nevertheless, drugs have been related to nosocomial ARF in up to 66% of patients older than 60 years.8 Drugs more commonly associated with nosocomial ARF are aminoglycosides, nonsteroidal anti-inflammatory drugs (NSAIDs), piperacillin-tazobactam, amphotericin B, combinations of trimethoprim with sulphonamides, cyclosporine, and angiotensin-converting enzyme inhibitors (ACEI).5
Several studies have described the frequency of ARF in patients admitted in medical and surgical departments,5,8–16 as well as that of drug-related ARF,5,8–13 but none have compared the characteristics and the morbi-mortality of patients with drug-related ARF to patients vs ARF of other causes.
The main purpose of this study was to determine the incidence of drug-related ARF in hospitalised patients, describe the most common drugs causing ARF, associated risk factors, morbi-mortality, and compare these characteristics with those observed in non-drug related ARF.
MethodsA prospective observational study was conducted in patients who developed ARF during admission in a tertiary hospital in Barcelona, between July 19th 2010 and July 31st 2011. Patients were monitored until hospital discharge.
The inclusion criteria were adult patients with an increase in serum creatinine of more than 0.5mg/dl observed in two consecutive measurements during hospitalisation in medical or surgical departments, if baseline concentration was ≤2.5mg/dl, or an increase in serum creatinine of more that 20%, in those patients with basal serum creatinine greater than 2.5mg/dl.1,2 Patients with an episode ARF at different admissions were enrolled.
Exclusion criteria were as follows: patients with no plasma creatinine measurements available during hospitalisation and before screening of impaired renal function; patients admitted due to ARF, flare of IRC, or renal transplants; patients undergoing chronic dialysis; patients participating in clinical trials; and patients who did not give their consent. Patients admitted in traumatology, gynaecology and obstetrics departments were also excluded, as well as patients with ARF episodes in critical care units (e.g. patients in intensive care unit (ICU) or patients with burns or bleeding) or in the post-surgery resuscitation room.
Baseline plasma creatinine was defined as mean creatinine from hospital admission and before meeting the inclusion criteria. Patients with CRF before admission were enrolled provided that plasma creatinine remained stable at the time of hospitalisation. Patients were screened via an electronic programme that provided a daily list of those who met the inclusion criteria according to laboratory data. Each case was validated by a nephrologist.
Based on electronic clinical records, data on patients’ demographics (age and gender), admission department, hospital stay, and medical history were collected. Information on ARF risk factors during admission (decompensated heart failure or clinical evidence of heart failure, low blood pressure or blood pressure below 90/60mmHg, bleeding or any cause of volume depletion as indicated in the clinical record) was also collected.
Data on the drugs administered during hospitalisation (active ingredients, dosing, and treatment duration) were collected using the electronic medical prescription sheet. Drugs were classified using the Anatomical, Therapeutic, Chemical (ATC) Classification System.17
Additionally, data on ARF complications during hospitalisation and discharge outcome (complete or partial recovery of renal function, no recovery at all, and need of renal replacement treatment) were collected. The severity of ARF was assessed based on the RIFLE classification (risk, damage, failure, prolonged loss of renal function, and final and irreversible failure of renal function).3 Data on discharge diagnoses were coded according to the International Disease Classification, Ninth Revision, Clinical Modification (ICD-9-CM).18
The analysis of drug accountability and the assessment of the causal relationship between drugs and ARF were conducted according to the methods and the algorithm of the Spanish System of Drug Surveillance (SEFV).19,20 These methods were used and agreed upon by 2 clinical pharmacologists were competent with its use. The assessment of the causal relationship included the time elapsed between treatment initiation and the onset of ARF, previous knowledge of the casual relationship between the drug and ARF, the effect of the dechallenge and rechallenge of the suspected drug, and other alternative causes of ARF. Accountability was determined based on the outcome of the causality algorithm as well as the existence of ARF risk factors. Categories of causality were defined as improbable, conditional, potential, probable or definite.19,20 Only patients with drug-related ARF who had at least one drug with definite, probable or potential were included in the analysis. All ARFs in which the association of the drugs prescribed during hospitalisation was conditional or improbable were considered non-drug related. ARF aetiology was thereby classified as pre-renal, renal, or post-renal according to the Spanish Society of Nephrology guidelines criteria.21
The incidence of nosocomial ARF (5–7%)1 and the number of hospitalisations in a year were taken into consideration in order to estimate the sample size. Two hundred and thirty-two patients with ARF during hospitalisation had to be enrolled in order to achieve a total of 20%7 drug-related ARFs during admission, with a 95% confidence interval (CI), and an accuracy of ±5%.
The study was conducted as per the national guidelines for post-authorisation studies and was approved by the hospital Clinical Research Ethics Committee.
The incidence (95% CI) of ARF among patients admitted in the permissible hospital departments was estimated based on ARF of any cause as a proportion of the total number of admissions in these departments during the study period. Patients complying with the inclusion criteria but did not give their consent were also included to estimate total incidence. The incidence (95% CI) of drug-related ARF was calculated by using drug-related ARF cases as the numerator.
Categorical variables were expressed as frequencies and proportions. Numeric variables were described as means±standard deviation (SD) or medians and interquartile ranges (IR). Proportions were compared using the Chi-square test. Means between the two groups were compared using the Student t-test for independent groups or its non-parametric alternative (Mann–Whitney U), based on each variable's distribution. A p value <0.05 was considered statistically significant. The statistical analysis was done through the statistical programme IBM SPSS Statistics version 20 (IBM Corp., New York, US).
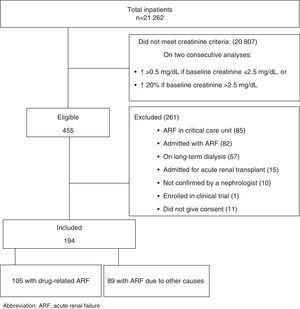
ResultsOne hundred and ninety-four cases of ARF during hospitalisation, out of a total of 21,262 admissions, were included during the study period (2 patients were admitted twice and developed an episode of ARF at each admission). A total of 20,807 admissions were excluded as they did not comply with the study inclusion criteria for creatinine abnormalities and 261 were excluded for other reasons (Fig. 1).
The incidence of ARF during hospitalisation and drug-related ARF was 9.6 per 1000 admissions (95% CI: 8.4–11) and 4.9 per 1000 admissions (CI 95%: 4–6), respectively. The incidence rate of ARF during hospitalisation was 1.4 per 1000 beds-days (95% CI: 1.2–1.6).
For nosocomial ARF the median age was 72 years (IR 20) and 60% of patients were male. The main demographics and the medical history of patients are described in Table 1.
Baseline characteristics.
| Characteristics | Drug-related ARF n=105 | Non-drug related ARF n=89 | p | Nosocomial ARF n=194 |
|---|---|---|---|---|
| Age, years, median [IR] | 72 [18] | 73 [22.5] | 0.86 | 72 [20] |
| >64 years, n (%) | 72 (68.6) | 57 (64.0) | 0.54 | 129 (66.5) |
| Gender, men, n (%) | 66 (63.0) | 51 (57.3) | 0.46 | 117 (60.0) |
| Medical history, n (%) | ||||
| High blood pressure | 78 (74.3) | 52 (58.4) | 0.02 | 130 (67.0) |
| Heart failure | 28 (26.7) | 13 (14.6) | 0.05 | 41 (21.1) |
| Ischaemic cardiopathy | 24 (23.0) | 12 (13.5) | 0.10 | 36 (18.6) |
| Diabetes mellitus | 42 (40.0) | 27 (30.3) | 0.17 | 69 (35.6) |
| Chronic renal failure | 37 (35.2) | 36 (40.4) | 0.46 | 73 (37.6) |
| Two or more | 63 (60.0) | 40 (45.0) | 0.04 | 103 (53.1) |
| Admitting service, n (%) | ||||
| Surgical | 53 (50.5) | 40 (44.9) | 0.47 | 93 (47.9) |
| Medical | 52 (49.5) | 49 (55.1) | 101 (52.1) | |
| Days from admission to ARF, mean (DE) | 11.8 (±9.1) | 12 (±14) | 0.56 | 11.9 (±11.9) |
| Creatinine prior to ARF, (mg/dl), mean (DE) | 1.24 (±0.46) | 1.29 (±0.69) | 0.95 | 1.26 (±0.58) |
| Maximum creatinine during ARF, (mg/dl), mean (DE) | 2.72 (±1.04) | 3.76 (±2.11) | <0.001 | 3.2 (±1.71) |
| Risk factors, n (%) | ||||
| Volume depletion | 40 (38.1) | 42 (47.2) | 0.24 | 82 (42.3) |
| Low blood pressure | 26 (24.8) | 35 (39.3) | 0.03 | 61 (31.4) |
| Decompensated heart failure | 20 (19.0) | 17 (19.1) | 1.00 | 37 (19.1) |
| Bleeding (from surgery site or a different site) | 8 (7.6) | 14 (15.7) | 0.11 | 22 (11.3) |
| No. of Risk factors, n (%) | ||||
| One | 50 (47.6) | 43 (48.3) | 93 (47.9) | |
| Two | 29 (27.6) | 20 (22.5) | <0.001 | 49 (25.3) |
| Three or more | 10 (9.5) | 26 (29.2) | 36 (18.5) | |
ARF: acute renal failure; IR: inter-quartile range; SD: standard deviation.
P value was determined by using a Chi-square test or a T-test and a Mann–Whitney U-test.
A total of 47.9% of patients were admitted in the surgical department, whereas 52.1% of patients were admitted in the medical department. The most common diagnoses leading to admission included: neoplasia (n=58; 29.8%), infectious disease (n=18; 9.2%), and non-renal transplant (n=16; 8.2%). Mean hospital stay was 25.6±20.4 days and mean time from admission to compliance with the study inclusion criteria for ARF was 11.9±11.9 days.
A 50–99% increase in creatinine from baseline was reported in half of the cases. A risk of renal damage was observed in 29.9% of patients and 47.9% developed renal damage according to the RIFLE classification (Table 2). Hyperpotassaemia was the most common ARF-related complication during hospitalisation (25.8%), followed by decompensated heart failure (12.9%), arrhythmia (4.1%), and digestive bleeding (3.6%).
Severity of ARF.
| Severity | Drug-related ARF n=105 | Non-drug related ARF n=89 | p | Nosocomial ARF n=194 |
|---|---|---|---|---|
| Increased creatinine, n (%) | ||||
| ≥50% | 58 (55.2) | 38 (42.7) | 0.21 | 96 (49.5) |
| ≥100% | 28 (26.7) | 32 (36.0) | 60 (30.9) | |
| ≥200% | 19 (18.1) | 19 (21.3) | 38 (19.6) | |
| RIFLE classification, n (%) | ||||
| Risk | 39 (37.1) | 19 (21.3) | 0.05 | 58 (29.9) |
| Injury | 46 (43.8) | 47 (52.8) | 93 (47.9) | |
| Failure | 20 (19.0) | 23 (25.8) | 43 (22.2) | |
ARF: acute renal failure.
P value was determined using a Chi-square test.
Complete, partial or incomplete recovery of renal function at discharge was reported in 95 (62.1%), 39 (25.5%), and 19 (12.4%) patients, respectively. Eight (4.1%) patients required haemodialysis, which was continued in 2 patients after discharge. Mortality during hospitalisation in patients with ARF was of 22.4%. For patients with a fatal outcome, death was related to ARF in 11 (5.7%) patients.
Impaired renal function was unrelated to drugs in 89 (45.9%) patients. Aetiology was pre-renal in 74 (83.1%) patients, renal in 10 (11.3%), and post-renal in 5 (5.6%) (Fig. 2).
Impaired renal function was related to drugs in 105 (54.1%) patients (Fig. 1). Drug accountability was classified as possible, probable, and definite in 22 (21%), 70 (66.6%), and 13 (12.4%) patients, respectively. However, there were one or more risk factors in 84.7% of drug-related cases (Table 1).
There were 305 drugs involved in the 105 drug-related ARF cases. Subgroups of the most common drugs were diuretics (33.4%, mainly furosemide), agents acting on the renin–angiotensin system (17.3%, mainly enalapril), β-adrenergic blockers (7.2%, mainly bisoprolol), immunosuppressors (7.5%, mainly tacrolimus), iodinated contrast (5.9%), and NSAIDs (5.5%) (Table 3). ARF was related to a single suspected drug in 20.0% of cases (diuretics and radio imaging contrasts were the most common, followed by ACEIs and NSAIDs), with 2 drugs in 22%, 3 drugs in 24%, 4 in 18%, and 5 or more in 16% of cases. The most common concomitant suspected drugs were diuretics and an agent acting on the renin–angiotensin system, alone or in combination with a third drug or more suspected drugs (36 cases, 34.3%).
Treatment groups, subgroups, and drugs related to ARF.
| Treatment groups n=305 (%) | Subgroups n=305 (%) | Drugs n=305 (%) | |
|---|---|---|---|
| Cardiovascular system 221 (72.4) | Diuretics 102 (33.4) | Furosemide | 70 (22.9) |
| Hydrochlorothiazide | 19 (6.2) | ||
| Spironolactone | 13 (4.3) | ||
| Active agents in the renin–angiotensin system 53 (17.4) | Enalapril | 23 (7.5) | |
| Losartan | 17 (5.6) | ||
| Captopril | 13 (4.3) | ||
| Beta-blockers 22 (7.2) | Bisoprolol | 6 (1.9) | |
| Labetalol | 4 (1.3) | ||
| Atenolol | 4 (1.3) | ||
| Carvedilol | 3 (1.0) | ||
| Nadolol | 3 (1.0) | ||
| Propranolol | 2 (0.7) | ||
| Calcium-channel blockers 21 (6.9) | Amlodipine | 14 (4.6) | |
| Nifedipine | 5 (1.7) | ||
| Diltiazem | 1 (0.3) | ||
| Nimodipine | 1 (0.3) | ||
| Cardiac therapy 15 (4.9) | Nitroglycerine | 11 (3.6) | |
| Isosorbide mononitrate | 2 (0.7) | ||
| Ivabradine | 1 (0.3) | ||
| Amiodarone | 1 (0.3) | ||
| Cardiovascular system 221 (72.4) | Anti-hypertensives 8 (2.6) | Doxazosin | 7 (2.3) |
| Hydralazine | 1 (0.3) | ||
| Antineoplastic and immunomodulating agents 30 (9.8) | Immunosuppressors 23 (7.5) | Tacrolimus | 16 (5.1) |
| Mycophenolate mofetil | 3 (1.0) | ||
| Cyclosporine | 2 (0.7) | ||
| Everolimus | 2 (0.7) | ||
| Anti-neoplastic agents 7 (2.3) | Citarabine | 2 (0.7) | |
| Carmustine | 1 (0.3) | ||
| Rituximab | 1 (0.3) | ||
| Melfalan | 1 (0.3) | ||
| Bortezomib | 1 (0.3) | ||
| Etoposide | 1 (0.3) | ||
| Several 18 (5.9) | Radiocontrast agents 18 (5.9) | Ioversol | 7 (2.3) |
| Iobitridol | 3 (1.0) | ||
| Iomeprol | 2 (0.7) | ||
| Iohexol | 1 (0.3) | ||
| Iopamidol | 3 (1.0) | ||
| Iopromide | 2 (0.7) | ||
| Musculoskeletal system 17 (5.5) | NSAIDs 17 (5.5) | Dexketoprofen | 16 (5.2) |
| Ibuprofen | 1 (0.3) | ||
| Systemic anti-infective agents 10 (3.3) | Antibacterials 4 (1.3) | Vancomycin | 2 (0.7) |
| Trimethoprim-Sulfamethoxazole | 2 (0.7) | ||
| Antifungals 3 (1.0) Antivirals 3 (1.0) | amphotericin b | 3 (1.0) | |
| Acyclovir | 1 (0.3) | ||
| Ganciclovir | 1 (0.3) | ||
| Valganciclovir | 1 (0.3) | ||
| Blood and haematopoietic organs 5 (1.6) | Platelet aggregation inhibitors 5 (1.6) | Acetylsalicylic Acid | 5 (1.6) |
| Urogenital tract and sex hormones 3 (1.0) | Alpha-adrenergic antagonists 3 (1.0) | Tamsulosin | 3 (1.0) |
| Nervous system 1 (0.3) | Antiepileptics 1 (0.3) | Pregabalin | 1 (0.3) |
ARF: acute renal failure; NSAID: nonsteroidal anti-inflammatory drugs.
Patients with drug-related ARF more commonly had a history of two or more previous pathological events, as well as history of high blood pressure and heart failure compared to patients with non-drug related ARF (Table 1). However, they less often had three or more risk factors of ARF and low blood pressure and in turn, they often had three or more risk factors of ARF and low blood pressure, and mean maximum creatinine was lower (Table 1). Furthermore, mortality during hospitalisation was lower among patients with drug-related ARF than in patients with ARF related to other causes (7.7% compared to 39.3%; p<0.001). The most common cause of death in both groups was neoplasia. Four patients with drug-related ARF and 7 patients with ARF related to other causes died of ARF.
DiscussionIn our study, 10 in every 1000 admissions developed an episode of ARF during hospitalisation and the episode was related to drugs in half of the cases. Drugs were the only risk factor in only 15% of drug-related cases; the remaining patients also had one or more risk factors. The most commonly used drugs were diuretics and ACEI or angiotensin receptor antagonists (ARA II), followed by β-adrenergic blockers and calcium channel blockers, immunosuppressors, contrast means, and NSAIDs. Patients who developed drug-related ARF during admission more commonly had a history of two or more previous pathological events; in turn, they had less risk factors of ARF, lower creatinine levels during the episode, and lower mortality. The frequency of nosocomial ARF5,8–15 and their aetiology, including drug-related ARF, have been described in several studies.5,8–13 However, this study uses an algorithm to determine the causal relationship between drugs and ARF and provides a detailed description of the characteristics of patients with drug-related ARF.
The incidence of nosocomial ARF described in studies has been variable, ranging from 0.16% in the Liaño et al. study,15 to 22.7% reported by Wang et al.16 The incidence observed in our study was similar to that described by Kohli et al.8 (1.4%), Lauzurica et al.11 (1.6%), and Shusterman et al. (1.9%)10 These differences in the incidence of ARF reported in studies may result from the various ARF definitions used, and from the modified inclusion criteria. In the Barrantes et al. trial,13 the incidence of ARF was higher (12.6%) compared to our study despite inclusion and exclusion criteria being similar. Nevertheless, a wider definition of ARF (increased creatinine of 0.3mg/dl or higher in any 48h-period) was used and incidence was estimated based on eligible patients rather than on the total number of admissions.
In the present study, nosocomial ARF patients were older, and there were more male cases. Over 50% had a mean of 2 or more previous pathological events, and almost 40% had CRF. These characteristics are similar to those described in the cohort of patients with nosocomial ARF in the study of Barrantes et al.,13 although the proportion of patients with CRF in this study was even higher (52.1%) and women were more affected (53.6%). In the Kohli et al.8 and Liaño et al.15 studies from previous years, the mean age was lower, as was the frequency of CRF reported by Kohli et al.8 However, patients currently admitted in hospitals are older, they have more comorbidities, and more diagnostic and therapeutic procedures, with a potentially high risk of kidney injuries.22
Almost 80% of patients in our study were at risk of renal damage or developed renal damage during the episode of ARF as per the RIFLE classification (Table 2). The severity of ARF cannot be compared with that of other studies as they did not use the RIFLE classification; however, the proportion of patients requiring dialysis in our study (4.1%) was lower than that described in other studies.5,8 This can be explained by the fact that no ARF episodes reported in cases admitted to critical care departments were included in our study.
Furthermore, mean hospital stay from patients in the study trebled that of patients admitted in general to hospital (8.7 days) during the study period. In several studies, mean hospital stay of patients with nosocomial ARF has been higher than that of patients without this complication.10,13 Nosocomial ARF was also associated with higher mortality,10,13 even in patients not requiring critical care.13 Mortality rates in patients with nosocomial ARF ranges from 10.8% in the Wang et al.16 study to 32% in the Hou et al. study.9 In our study the mortality was higher than the mortality described in the study by Barrantes et al. (14.8%), although the cause of most deaths was not ARF.
Use of drugs is known to result in ARF. Several authors have studied the most common drugs, the mechanisms, and predisposing factors involved in acquiring ARF.23–26 The frequency of drug-related ARF described in Kohli et al.8 and Barrantes et al. studies13 (66 and 72.3%, respectively) is similar to that from our study. In contrast, Nash et al.5 and Jha et al.12 describe a lower frequency (16 and 29%, respectively). Only nosocomial ARF episodes were considered in all of these studies, and ARF episodes from patients admitted in the ICU were excluded in the Barrantes et al. study only,13 similar to our study.
Diuretics, followed by ACEI and ARA II, have been more commonly associated with ARF episodes. Concomitant use of diuretics and renin–angiotensin antagonists, alone or with other drugs, have been reported in almost 40% of drug-related ARF episodes in our study. Each of these drugs may affect renal function due to several mechanisms and, when used in combination, can lead to increased risk of acute renal injury.27,28 NSAIDs are renown nephrotoxic agents.29 Increased risk of acute renal injury has been described in a recent study when NSAIDs are added to the treatment with at least 2 of the following antihypertensive drugs: diuretics, ACEI or ARA II.30 NSAIDs5,8,13 as well as ACEI5,8,13 and diuretics11 have also been related to nosocomial ARF in other studies. Unlike a number of previous studies,5,8–11 aminoglucosides have not been the main cause of drug-related ARF in this study, probably as a result of the lower use at present.31,32 In addtion, no ARF cases related to β-lactamic antibiotics or quinolones, often associated with interstitial nephritis, were reported23,33 in this study. In fact no cases of acute interstitial nephritis were identified in our study. Other drugs, including the anti-hypertensives such as amlodipine or bisoprolol, immunosuppressors such as tacrolimus, and contrast media, have also been related to ARF episodes. Tacrolimus, an immunosuppressor commonly used to prevent solid organ transplant rejection and to treat immune systemic diseases, is a known nephrotoxic drug.34,35 On the other hand, contrast media still result in impaired renal function in hospitals, in spite of recommended preventive measures.36–38
Medical history including high blood pressure or heart failure was more common among patients developing drug-related ARF during hospitalisation, and use of cardiovascular drugs were most commonly involved in ARF episodes during hospitalisation. Also, these patients are especially prone to impaired renal function during admission. In order to prevent ARF episodes in these patients special attention should be paid to dosing of cardiovascular drugs, especially diuretics and other anti-hypertensives. Also certain combinations, including NSAIDs, should be restricted. Moreover, some risk factors, including volume depletion, dehydration, and low blood pressure during hospitalisation, should be avoided where possible. Most patients developing a drug-induced ARF episode in our study had 1 or 2 concomitant risk factors. In the Kohli et al. study most drug-related cases were also accompanied by other risk factors.8
This study has a number of limitations. Even though it is a single-centre study, we think the results may be extrapolated to other hospitals of similar characteristics. The study took place in a tertiary hospital with all medical and surgical specialties available, and the level of complexity is similar to that of other hospitals sharing the same features.
The clinical records of the cases were used to obtain the ARF risk factors. In light of this, some data may have been omitted, although this data was not clinically relevant.
The strengths of our study lie in the fact that it has a prospective design and that impaired renal function was screened via an electronic programme. This allowed for a reliable estimate of the incidence of nosocomial ARF. Also, a standardised and reproducible method was used to determine the causal relationship between drugs and ARF episodes.
In conclusion, half of the ARF episodes developed during hospitalisation in medical and surgical departments were drug-related in our study. The most common drugs used for the treatment of cardiovascular diseases, included diuretics or renin–angiotensin inhibitors, or others like immunosuppressors, contrast media, and NSAIDs. In addition, patients with drug-related ARF episodes were more prone to have cardiovascular events. In order to avoid ARF episodes during hospitalisation among these patients, these drugs should be carefully dosed and certain drug combinations should be avoided, while paying special attention to the presence of potential risk factors.
Conflicts of interestThe authors have no conflicts of interest to declare.
This research was funded by grant no. 374/09/08 provided by a public call for granting of subsidies for research projects conducted in clinical and health services in 2008 (Catalan Health Department and Catalan Agency for Health Technology Assessment and Research in DOGC No 5464, September 2009).
Please cite this article as: Iavecchia L, Cereza García G, Sabaté Gallego M, Vidal Guitart X, Ramos Terrades N, de la Torre J, et al. Insuficiencia renal aguda relacionada con medicamentos en pacientes hospitalizados. Nefrologia. 2015;35:523–532.