Pediatric chronic kidney disease (CKD) has peculiar features. In particular, growth impairment is a major clinical manifestation of CKD that debuts in pediatric age because it presents in a large proportion of infants and children with CKD and has a profound impact on the self-esteem and social integration of the stunted patients. Several factors associated with CKD may lead to growth retardation by interfering with the normal physiology of growth plate, the organ where longitudinal growth rate takes place. The study of growth plate is hardly possible in humans and justifies the use of animal models. Young rats made uremic by 5/6 nephrectomy have been widely used as a model to investigate growth retardation in CKD. This article examines the characteristics of this model and analyzes the utilization of CKD induced by high adenine diet as an alternative research protocol.
La enfermedad renal crónica (ERC) tiene características específicas. De manera especial, el retraso del crecimiento es una manifestación clínica importante de la ERC que se inicia en la infancia ya que se presenta en un gran número de lactantes y niños con ERC, y repercute profundamente en la autoestima e integración social de los pacientes afectados. Varios factores asociados con la ERC pueden provocar retraso del crecimiento por interferencia con la fisiología normal de la placa de crecimiento, el órgano donde se produce el ritmo de crecimiento longitudinal. Apenas es posible estudiar la placa de crecimiento en seres humanos y ello justifica el uso de modelos animales. El modelo más utilizado para investigar el retraso del crecimiento en la ERC son ratas jóvenes que se convierten en urémicas por nefrectomía 5/6. Este artículo revisa las características de este modelo y analiza el uso de la ERC inducida por una dieta con elevado contenido de adenina como protocolo de investigación alternativo.
Chronic kidney disease (CKD) is a global public health problem because of its associated adverse health outcomes and high healthcare costs. The Disease Fact Sheet 2014 published by the Centers for Disease Control and Prevention (CDC), estimates that more than 10% of adults in USA, which accounts for more than 20 million people only in this country, have CKD with variable disease seriousness. Diabetes and hypertension are responsible for 7 out of 10 new cases of end stage renal disease (ESRD) in USA. From 1990 to 2010, deaths from CKD raised by about 82% worldwide accounting for the third largest increase among the top 25 causes of death, after acquired immunodeficiency syndrome (396%) and diabetes (93%).1 Cardiovascular complications are the leading cause of mortality in adults with CKD so that these patients are more prone to die from cardiovascular events than to reach ESRD.
Peculiarities of pediatric chronic kidney diseaseAs adults represent the vast majority of CKD patients, most publications on CKD focus on adult population. However, CKD that presents at pediatric age, although less prevalent in absolute terms, has important and distinct peculiarities, as briefly commented below.
DemographyWhereas the prevalence of CKD in adults is well known, there are scarce reliable data in children. In North America, 11 cases per million of diagnosed CKD in children between 0 and 19 years have been reported, the prevalence being higher in males and blacks. In the European Union the incidence stands around 11–12 cases per million of the age-related population and the prevalence 56–75 cases per million of the age-related population, according to several national registries.2,3
CausesThe causes of CKD in pediatric population also differ from those of adults. Congenital anomalies of the kidney and urinary tract (CAKUT) are the leading cause and account for approximately 50% of cases. By contrast, acquired glomerulonephritis are the cause of CKD only in 5–14% of children although this percentage is higher in adolescents and ESRD cases.4 In European registries, the proportion of CAKUT (58–59%) was slightly higher, while the proportion of glomerulonephritis was lower (5–7%) than in the NAPRTCS database.5,6
Clinical manifestationsThe key role of interstitial nephropathies as responsible for pediatric CKD explains that the majority of children who have CKD, and even ESRD, are polyuric or have preserved diuresis, unlike adults. This facilitates the management of these patients but also entails peculiar therapeutic implications because of the poorly regulated loss of water and electrolytes, sodium in particular.It is also of note that CKD is often present since the first months of life and lasts until adulthood.
Although the final height of CKD patients has improved over the last decades,7 the North American Pediatric Trials and Collaborative Studies (NAPRTCS) 2008 Annual Report showed that the mean height of 7037 pediatric CKD patients was −1.44 SD score (SDS) and 35% of children had a height below −2 SD.4 Similar data shows the Spanish national registry of children with CKD (REPIR II), where the mean height of 605 patients was −1.03 SD and 25% of the children had a height below −1.88 SD8.
On the other hand, a variety of neurocognitive deficits occur in children with CKD.9 Thus, pediatric CKD has a profound impact on somatic growth, bone metabolism, and neurocognitive development.10 The CKD-related effects and its long-term sequelae are to a large extent different from those found in adult patients.
TreatmentAn adequate metabolic control, optimal nutritional management, appropriate hormonal therapy, intensive dialysis and early renal transplantation are the best remedies to improve growth and neurological development of children with CKD.11
Animal modelsAnimal models have long been used as a research strategy to increase the knowledge of diseases that affect humans, particularly to better understand pathophysiological mechanisms and to test new therapies. There is wide agreement that the best experimental models for the study of human disease should mimic the entity under consideration in terms of anatomy, physiology, and course of the disease. Thus, useful experimental models should facilitate studies both as the disease evolves and in stable chronic disease. Further, a useful animal model must adhere to current animal welfare regulations and needs to be technically feasible and financially sustainable. Therefore, it should be reproducible, simple, with brief experimental time and easy interpretation of the results. Even if the animal model meets these criteria, it is of note that findings derived from animal models must be taken with caution at the moment of establishing a translation of the results to clinical practice.12
Several types of animal models can be used. Over the last few decades the use of genetically modified mice has progressively increased mostly to figure out the function of a gene or a protein and, in turn, to clarify the pathogenesis and/or the pathophysiological mechanism of a given disease. Non-genetic models utilize infusions of drugs/substances, manipulation of diet or environment, or surgical procedures to induce the target disease. Depending upon the aims of the intended research, such models may be important and necessary. The use of inbred animals, which has been common for over 50 years,13 is of special interest to preserve particular traits and to study a hereditary disease in young animals.
Rats and mice are most frequently used as animal models of human disease because these species are easy to handle, relatively cheap and, as a result of their quick life cycle, the effects of manipulations or therapeutic trials can usually be appreciated in a short period of time. Moreover, the rodent animal model is able to reproduce the metabolic disorders and bone disease that patients with CKD develop during the clinical course of the disease.14–16
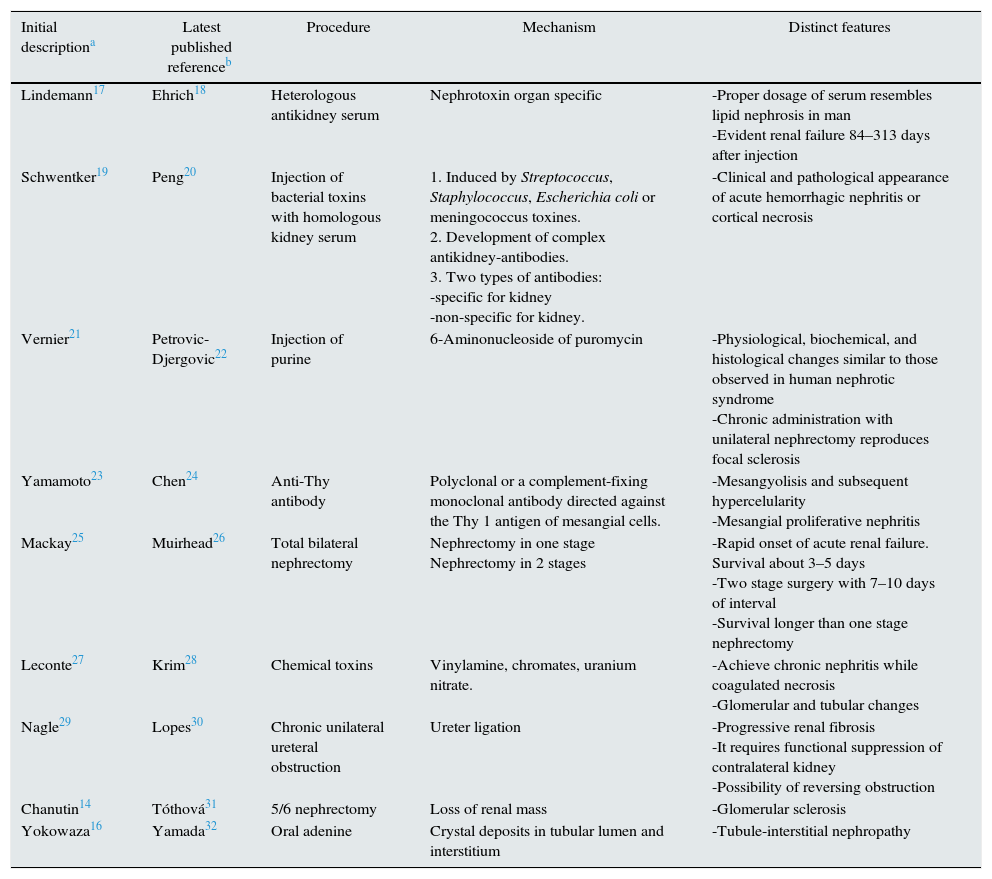
Models of CKDTable 1 shows CKD rat models used to clarify the pathogenesis and pathophysiology of kidney disease and the response to several types of therapeutic strategies. Table 1 informs on the first publication describing the model, the latest publication based on the model at the time of writing this manuscript, methodology, mechanism by which renal failure is reached and characteristic features.
Models of chronic kidney disease (CKD) developed in rats.14,16–32
| Initial descriptiona | Latest published referenceb | Procedure | Mechanism | Distinct features |
|---|---|---|---|---|
| Lindemann17 | Ehrich18 | Heterologous antikidney serum | Nephrotoxin organ specific | -Proper dosage of serum resembles lipid nephrosis in man -Evident renal failure 84–313 days after injection |
| Schwentker19 | Peng20 | Injection of bacterial toxins with homologous kidney serum | 1. Induced by Streptococcus, Staphylococcus, Escherichia coli or meningococcus toxines. 2. Development of complex antikidney-antibodies. 3. Two types of antibodies: -specific for kidney -non-specific for kidney. | -Clinical and pathological appearance of acute hemorrhagic nephritis or cortical necrosis |
| Vernier21 | Petrovic-Djergovic22 | Injection of purine | 6-Aminonucleoside of puromycin | -Physiological, biochemical, and histological changes similar to those observed in human nephrotic syndrome -Chronic administration with unilateral nephrectomy reproduces focal sclerosis |
| Yamamoto23 | Chen24 | Anti-Thy antibody | Polyclonal or a complement-fixing monoclonal antibody directed against the Thy 1 antigen of mesangial cells. | -Mesangyolisis and subsequent hypercelularity -Mesangial proliferative nephritis |
| Mackay25 | Muirhead26 | Total bilateral nephrectomy | Nephrectomy in one stage Nephrectomy in 2 stages | -Rapid onset of acute renal failure. Survival about 3–5 days -Two stage surgery with 7–10 days of interval -Survival longer than one stage nephrectomy |
| Leconte27 | Krim28 | Chemical toxins | Vinylamine, chromates, uranium nitrate. | -Achieve chronic nephritis while coagulated necrosis -Glomerular and tubular changes |
| Nagle29 | Lopes30 | Chronic unilateral ureteral obstruction | Ureter ligation | -Progressive renal fibrosis -It requires functional suppression of contralateral kidney -Possibility of reversing obstruction |
| Chanutin14 | Tóthová31 | 5/6 nephrectomy | Loss of renal mass | -Glomerular sclerosis |
| Yokowaza16 | Yamada32 | Oral adenine | Crystal deposits in tubular lumen and interstitium | -Tubule-interstitial nephropathy |
This manuscript focuses on the 5/6 nephrectomy uremic model, because it has been the most widely used to analyze several aspects of CKD in young rats, particularly those related with longitudinal growth and bone metabolism, and on the model of CKD induced by adenine and described in adult rats, because this model might be useful as animal model of pediatric CKD and presents significant advantages on the subtotal nephrectomy.
The 5/6 nephrectomy modelSubtotal nephrectomy in two stages, usually 4–7 days apart, to induce CKD was first described by Chanutin and Ferris14 in 1932. In the first stage, the upper and lower poles of the left kidney are functionally suppressed either by surgical scission, by ligation of the renal artery branches or by electrocauterization of renal cortex. In the second stage, the entire right kidney is removed giving rise to the final 5/6 elimination of renal mass, approximately. After the two stages, animals become immediately uremic. According to the original description14 urea blood concentration decreases to a constant level during the first 10 days followed by a gradual increment when animals initiate polyuria with early dehydration and loss of weight. Posterior investigations showed loss of weight in uremic rats compared to sham operated animals.33 In 1974, Chantler et al.,15 used 5/6 nephrectomy as a model of growth retardation secondary to CKD and described anorexia as a characteristic feature of the experimental CKD so that when glomerular filtration rate drops to below 50% of normal values, uremic rats eat 30% less than control group. This finding justified the need of using sham-operated, normal renal function rats pair fed with the uremic ones to distinguish between the effects of uremia per se from those induced by the associated malnutrition.
The adenine modelThe deficit of adenine phosphoribosyltransferase is a rare inherited metabolic disorder that impairs the formation of 2,8-dihydroxyadenine (DHA) in urine.34 Low solubility of DHA results in recurrent urolithiasis and nephropathy secondary to crystal precipitation into renal parenchyma.35 Adenine is more extensively retained in body tissues than the other dietary purines orally consumed (guanine, hypoxantine and xantine).36 High oral administration of adenine is immediately metabolized to DHA, which precipitates and forms crystals in the microvilli and the apical epithelial region of the proximal tubule in just 2 days after the adenine intake.37 In 1982, Yokowaza et al.16 proposed a new renal failure model based on excessive intake of adenine. This model induces metabolic alterations reproducing CKD characterized by crystalline deposits, foreign body granulomas formation in the renal tubules, and interstitium, and marked fibrosis leading to tubule-interstitial disease, as usually occurs in many pediatric patients with CKD. Moreover, these animals develop hyperphosphatemia, secondary hyperparathyroidism, bone mineral disease, and vascular calcification.38 Recent data indicate that some chemokines (CCR5 and CCL3) play an important role in this CKD model by exacerbating the insult and promoting fibrosis of the tubule-interstitial lesions.39
Different degrees of uremia can be achieved by varying the concentration of adenine in diet. Most publications in adult rats use 0.75% adenine diet to develop this rat model. However, dietary protocols have not been well established, either in terms of the adenine concentration in chow, ranging from 0.075 to 0.75%, or the duration of adenine administration, up to 25 weeks.40 Observational periods and therapeutic strategies have also varied across studies.
The rat's gender influences on the response to the adenine protocol. In 2006, Origima et al.41 concluded that adenine diet concentration needs to be higher in female rats than in male rats to induce the same degree of renal failure. Moreover, they found that male rats fed with adenine diet presented lower levels of testosterone and reduced bone mineral density than those receiving normal chow probably because of a mechanism independent of the severity of renal failure. This could be explained by the fact that adenosine triphosphate and other nucleotides can stimulate directly the formation and resorptive activity of osteoclasts, as well as inhibit bone mineralization by osteoblasts. Thus, high doses of adenine lead to an increase in adenosine, which might have direct effects on the function of osteoblasts and osteoclasts.42 There are no differences between both genders with regard to increased blood pressure, ventricular stiffness or cardiac fibrosis.
The adenine model has been used for testing few treatments. Thus, Ali et al.43 demonstrated in a model of CKD caused by adenine the usefulness of Gum acacia (a variety of plant) to treat the anemia of CKD. In a posterior study, and using a 0.75% adenine model, they reported that gum acacia improved histological kidney alterations by reducing adenine deposits and reduced hypertrophy of myocardium.44 Tong et al.45 demonstrated a slight amelioration of renal function by administration of Chinese herbs or glibenclamide using a protocol of rats fed with adenine.
5/6 nephrectomy vs adenine modelsSome reports have shown that CKD induced by adenine intake results in a greater degree and extent of cardiovascular lesions in the intima-media of the carotid artery than CKD caused by 5/6 nephrectomy, for the same time duration of renal failure.46 Likewise, CKD rats receiving adenine develop significant bone mineral alterations earlier than 5/6 nephrectomized rats.47 In the adenine model, vascular calcification and left ventricular mass increased progressively with the duration of CKD in association with increased serum fibroblast growth factor 23 (FGF23) and high pulse pressure.48
Ferrari et al.49 recently reported the first comparative study between the adenine and the subtotal nephrectomy models in a 9 weeks’ protocol. Animals were submitted to a 0.75% adenine diet for the first 2 weeks and, subsequently, to a 0.5% adenine diet. The two groups were comparable in terms of circulating concentrations of ionized calcium, phosphate, parathyroid hormone, and FGF23. Despite the similar serum phosphate levels, fractional excretion of phosphorus was higher in the adenine group. These animals developed a more severe form of bone disease than rats with subtotal nephrectomy.
It is of note that when the 5/6 nephrectomy is carried out by excision of one kidney and infarction of the two poles of the remaining kidney by arterial ligation, there is a marked increased risk of renovascular hypertension which must be taken into account at the time of assessing the cardiovascular effects of this CKD model.50
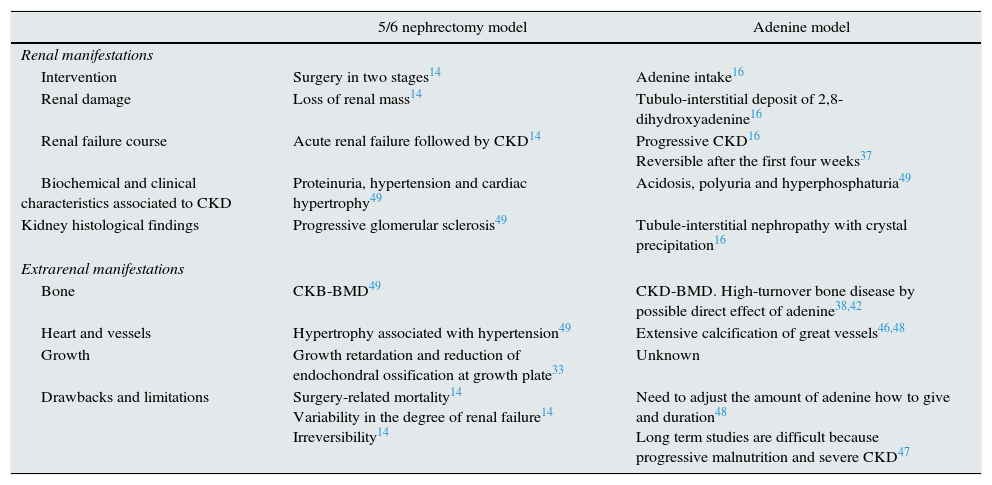
Table 2 summarizes the main characteristics of 5/6 nephrectomy and adenine models of CKD.
Characteristics of rat models of chronic kidney disease CKD induced by subtotal nephrectomy o adenine intake.14,16,33,37,38,42,46–49
| 5/6 nephrectomy model | Adenine model | |
|---|---|---|
| Renal manifestations | ||
| Intervention | Surgery in two stages14 | Adenine intake16 |
| Renal damage | Loss of renal mass14 | Tubulo-interstitial deposit of 2,8-dihydroxyadenine16 |
| Renal failure course | Acute renal failure followed by CKD14 | Progressive CKD16 Reversible after the first four weeks37 |
| Biochemical and clinical characteristics associated to CKD | Proteinuria, hypertension and cardiac hypertrophy49 | Acidosis, polyuria and hyperphosphaturia49 |
| Kidney histological findings | Progressive glomerular sclerosis49 | Tubule-interstitial nephropathy with crystal precipitation16 |
| Extrarenal manifestations | ||
| Bone | CKB-BMD49 | CKD-BMD. High-turnover bone disease by possible direct effect of adenine38,42 |
| Heart and vessels | Hypertrophy associated with hypertension49 | Extensive calcification of great vessels46,48 |
| Growth | Growth retardation and reduction of endochondral ossification at growth plate33 | Unknown |
| Drawbacks and limitations | Surgery-related mortality14 Variability in the degree of renal failure14 Irreversibility14 | Need to adjust the amount of adenine how to give and duration48 Long term studies are difficult because progressive malnutrition and severe CKD47 |
CKD, chronic kidney disease; CKD-BMD, CKD-bone mineral disorders.
The adenine model of CKD is easy to do and reproducible. It does not require invasive interventions and results in tubule-interstitial lesions similar to those of CKD that debuts in infancy and early-childhood. It is associated with the 100% of animal survival when the adenine diet concentration is adjusted and the induced CKD may be reversible at not very high adenine doses. In spite of these characteristics, there are no published studies of young rats made uremic by adenine administration.
We propose that administration of adenine to prepubertal rats might be a suitable procedure for the study of CKD in young individuals. Further studies are needed to characterize this model in young rats.
FundingThis study has been supported partially by Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (PI12/00987 y PI15/02122), National Plan 2013–2016, Fondos FEDER, University of Oviedo and Fundación Nutrición y Crecimiento.
Conflict of interestThe authors declare no conflict of interest.