The Kidney Donor Profile Index (KDPI), together with other donor and recipient variables, can optimise the organ allocation process. This study aims to check the feasibility of the KDPI for a Spanish population and its predictive ability of graft and patient survival.
Materials and methodsData from 2734 kidney transplants carried out in Andalusia between January 2006 and December 2015 were studied. Cases were grouped by recipient age, categorised by KDPI quartile and both graft and patient survival were compared among groups.
ResultsThe KDPI accurately discriminated optimal organs from suboptimal or marginal ones. For adult recipients (aged: 18–59 years) it presents a hazard ratio of 1.013 (P<0.001) for death-censored graft survival and of 1.013 (P=0.007) for patient survival. For elderly recipients (aged: 60+ years), KDPI presented a hazard ratio of 1.016 (P=0.001) for death-censored graft survival and of 1.011 (P=0.0007) for patient survival. A multivariate analysis identified the KDPI, donor age, donation after circulatory death, recipient age and gender as predictive factors of graft survival.
ConclusionsThe results obtained show that the KDPI makes it possible to relate the donor's characteristics with the greater or lesser survival of the graft and the patient in the Spanish population. However, due to certain limitations, a new index for Spain based on Spanish or European data should be created. In this study, some predictive factors of graft survival are identified that may serve as a first step in this path.
El Kidney Donor Profile Index (KDPI), junto a otras variables del donante y receptor, puede optimizar el proceso de asignación de órganos. Este estudio tiene como objetivo comprobar la aplicabilidad del KDPI en una población española, así como su capacidad de predicción de la supervivencia del injerto y del paciente.
Materiales y métodosSe estudiaron 2.734 trasplantes renales llevados a cabo en Andalucía entre enero de 2006 y diciembre de 2015. Los casos se agruparon por edad del receptor y cuartil del KDPI y se compararon entre grupos tanto la supervivencia del injerto como la del paciente.
ResultadosEl KDPI discrimina con precisión los órganos óptimos de los subóptimos o marginales. Para receptores entre 18 y 59años presenta un hazard ratio de 1,013 (p<0,001) para supervivencia de injerto censurada para muerte y de 1,013 (p=0,007) para supervivencia del paciente. Para receptores mayores de 60años el hazard ratio es de 1,016 (p=0,001) para supervivencia del injerto censurada para muerte y de 1,011 (p=0,007) para supervivencia del paciente. Un análisis multivariante identificó como factores predictivos de la supervivencia del injerto el KDPI, la edad del donante, la donación tras muerte circulatoria, la edad y el sexo del receptor.
ConclusionesEl KDPI permite relacionar, a grandes rasgos, las características del donante con la mayor o menor supervivencia del injerto y del paciente en la población española. No obstante, debido a ciertas limitaciones, convendría elaborar un índice propio a partir de los datos españoles o europeos. En este trabajo se identifican algunos factores predictivos de la supervivencia del injerto que pueden servir como primer paso en esa línea.
End-stage renal disease (ESRD) is an increasing worldwide health concern, and kidney transplantation is the best treatment for survival and quality of life of people with ESRD.1,2 The ageing of donors (currently achieving a mean of 59.2 years in Spain3) and the high discarding rate of organs by biopsy4 are among the main obstacles for transplantation. Performing a right assessment of kidney quality and feasibility is a current challenge to reduce discarding rate of organs potentially valid.
There are several methods to assess the quality of kidneys5 and, for organ allocation, the Kidney Donor Risk Index (KDRI) has acquired special relevance. The KDRI combines 14 donor and transplant factors and provides an estimation of the relative risk of graft failure after kidney transplant from a deceased donor compared to the reference donor.6 Although its discriminatory power is moderate, the KDRI represents a step forward in pretransplantation kidney assessment and allograft prediction.
A new allocation policy based on the KDRI was implemented by the end of 2014 in the United States. Since some transplant factors are generally unknown at the time offer is made, this policy uses a new index, the Kidney Donor Profile Index (KDPI)7 based on the donor-only KDRI version including 10 donor factors. The KDPI is the numerical ranking of the organ to be evaluated compared to all kidneys recovered the previous year. During the allocation process, the KDPI is an aid tool to decide whether to accept an offer of a deceased donor kidney, often discarding organs with KDPI>85% (considered high risk donors).
Last 10 years in Spain, and concretely in Andalucía, the acceptance of a kidney from an expanded criteria donor (ECD)8 has primary based on the result of preimplantation biopsy.9 But biopsy continues having a controversial role on the assessment of renal graft feasibility. Meanwhile some studies justify its use specially for double transplant,10 others doubt about its prediction capacity for renal functioning.11–13
Using a risk index (such as the KDPI) could help the decision-making in Spain for ECD cases when decision depends on the results of the biopsy. Several studies support this hypothesis. Querard et al.8 showed that the relative differences between ECD and standard criteria donors (SCD) were lower in Europe than in North America, particularly for death-censored graft failure. Another study concluded that high-KDPI transplantation offers a better survival versus remaining on the waitlist for recipients older than 50 years when wait time is higher than 33 months.14
This study aims: (1) to check the adequacy of the KDPI to discriminate differences on graft and patient survival for Spain recipients, and (2) to identify predictor factors of graft survival that could contribute to create a Spanish or European index.
Materials and methodsA retrospective analysis was performed on data from a database held by the Regional Transplant Coordination of Andalusia (SICATA). Data were retrieved for single kidney transplants performed between January 2006 and December 2015. Graft failure was defined by return to chronic maintenance dialysis. The final follow-up date was 31 December 2015. Statistical analyses were performed using SPSS 24.0 (IBM SPSS Statistics for Windows, Version 24.0. IBM Corp., Armonk, NY, USA).
Since in Spain it is no possible to calculate KDPI as in US, our approach is to use the study population itself to map KDPI from donor factors (see Section Discussion for a comment about this limitation). Cases were ranked, categorised in quartile, and compared for graft survival (death-censored and uncensored) and patient survival using Kaplan–Meier log-rank survival analysis. The associations between the KDPI as a continuous variable and survival of graft and patient were determined using Cox Regression univariate analysis. Cases resulting on patient's death with functioning graft were excluded since this is a competitive event for graft failure and it may alter the results. Furthermore, the discriminative ability of the KDPI was tested with Harrell's C stat for Cox models.
Cox multivariate regression was used for identifying predictor factors of graft survival. Recipient risk factors significantly associated with graft failure using univariable screening at the level of P<0.05 were included in the final multivariable model. These were age, gender, height, diabetes as primary renal disease, time on renal replacement therapy (RRT), HIV and HCV status. Donor variables included HLA mismatch and elements conforming KDPI. Since ethnicity of Spain population is highly uniform, donor and recipient race were not included.
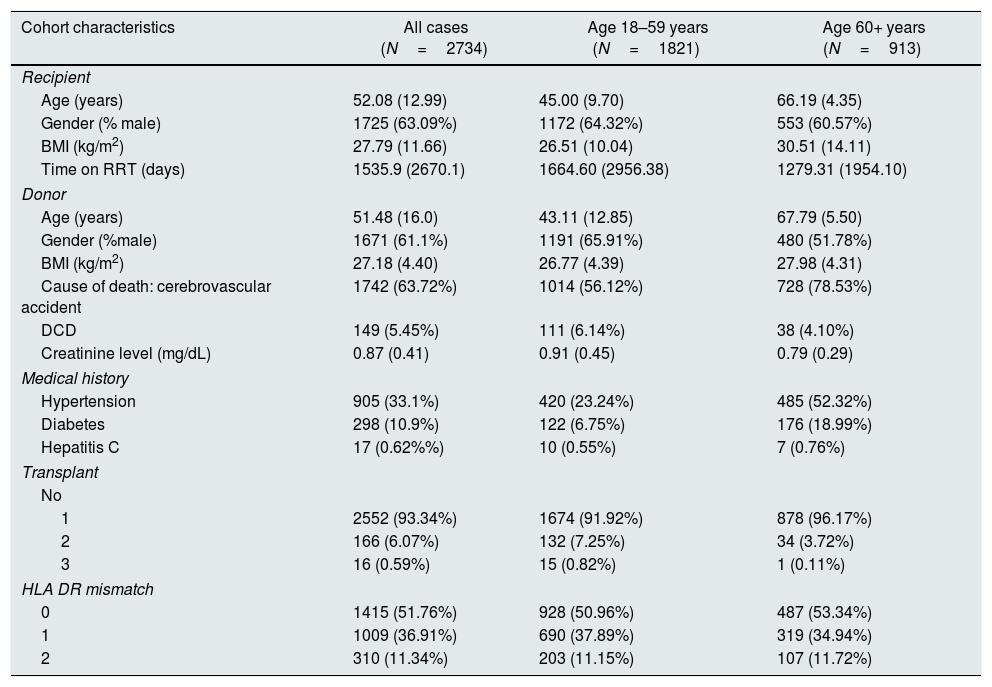
ResultsDemographicsData for 3406 kidney transplants were retrieved. A total of 672 cases were excluded due to missing data, and 2734 kidney transplants were included in the analysis. The demographics of the recipient and donor cohort are displayed in Table 1. As the SICATA does not record ethnicity, we classified all recipients in our population as Caucasian.
Recipient and donor characteristics.
| Cohort characteristics | All cases (N=2734) | Age 18–59 years (N=1821) | Age 60+ years (N=913) |
|---|---|---|---|
| Recipient | |||
| Age (years) | 52.08 (12.99) | 45.00 (9.70) | 66.19 (4.35) |
| Gender (% male) | 1725 (63.09%) | 1172 (64.32%) | 553 (60.57%) |
| BMI (kg/m2) | 27.79 (11.66) | 26.51 (10.04) | 30.51 (14.11) |
| Time on RRT (days) | 1535.9 (2670.1) | 1664.60 (2956.38) | 1279.31 (1954.10) |
| Donor | |||
| Age (years) | 51.48 (16.0) | 43.11 (12.85) | 67.79 (5.50) |
| Gender (%male) | 1671 (61.1%) | 1191 (65.91%) | 480 (51.78%) |
| BMI (kg/m2) | 27.18 (4.40) | 26.77 (4.39) | 27.98 (4.31) |
| Cause of death: cerebrovascular accident | 1742 (63.72%) | 1014 (56.12%) | 728 (78.53%) |
| DCD | 149 (5.45%) | 111 (6.14%) | 38 (4.10%) |
| Creatinine level (mg/dL) | 0.87 (0.41) | 0.91 (0.45) | 0.79 (0.29) |
| Medical history | |||
| Hypertension | 905 (33.1%) | 420 (23.24%) | 485 (52.32%) |
| Diabetes | 298 (10.9%) | 122 (6.75%) | 176 (18.99%) |
| Hepatitis C | 17 (0.62%%) | 10 (0.55%) | 7 (0.76%) |
| Transplant | |||
| No | |||
| 1 | 2552 (93.34%) | 1674 (91.92%) | 878 (96.17%) |
| 2 | 166 (6.07%) | 132 (7.25%) | 34 (3.72%) |
| 3 | 16 (0.59%) | 15 (0.82%) | 1 (0.11%) |
| HLA DR mismatch | |||
| 0 | 1415 (51.76%) | 928 (50.96%) | 487 (53.34%) |
| 1 | 1009 (36.91%) | 690 (37.89%) | 319 (34.94%) |
| 2 | 310 (11.34%) | 203 (11.15%) | 107 (11.72%) |
Data are presented as mean (SD) or n (%). BMI, body mass index; DCD, donor after circulatory death (all categories included); HLA, human leucocyte antigen; RRT, renal replacement therapy.
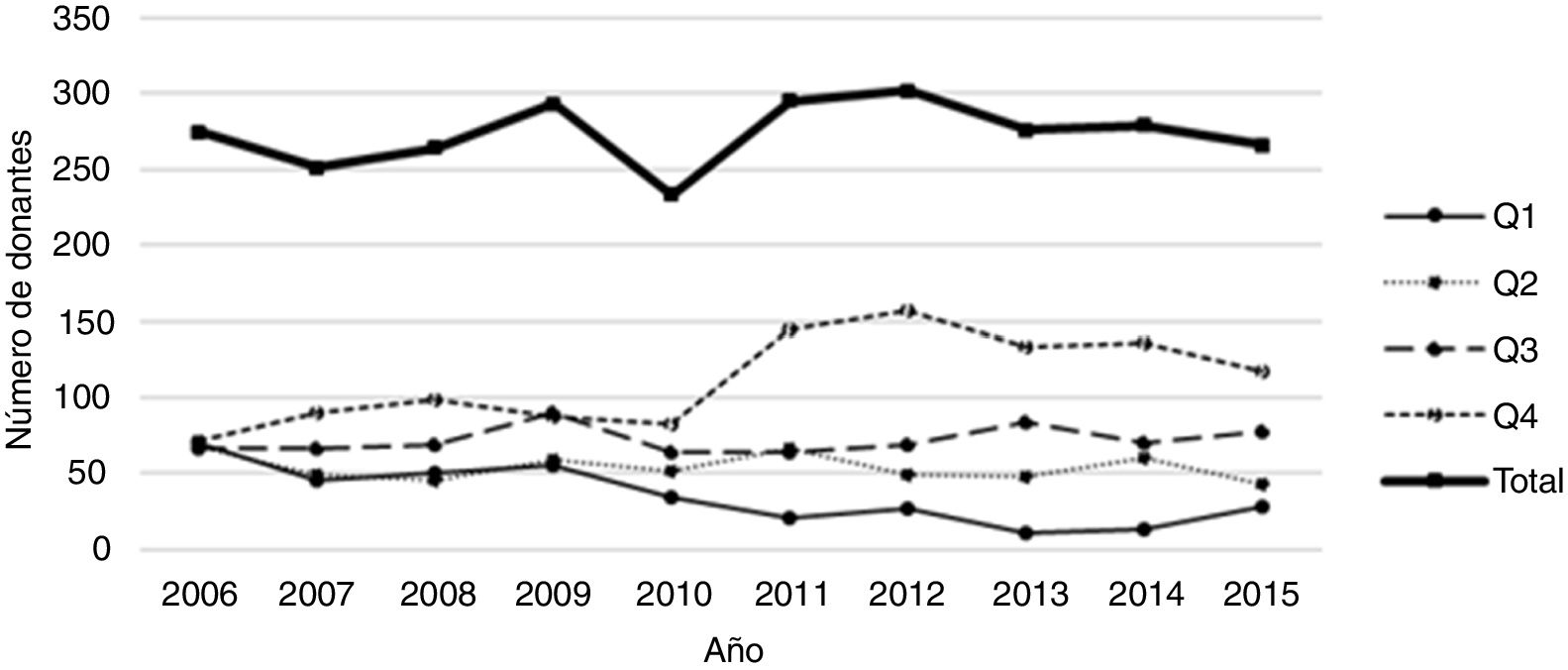
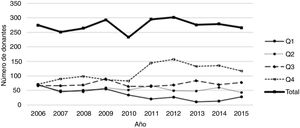
As mentioned, KDPI was calculated by using the own study population, and cases were grouped by quartiles. Fig. 1 shows the evolution of the number of organs in each quartile for this population during the inclusion period. The number of organs from the first quartile (i.e., those with the best characteristics) decreases, meanwhile the number of organs in the fourth quartile (the one with the worst prognosis) increases. This is a consequence of the greater use and acceptance of organs with, a priori, worse characteristics (for example, from old donors).
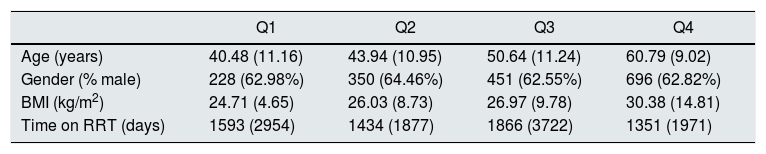
Table 2 shows the recipients classified according to KDPI quartile of the received organ. In the comparison of characteristics between subpopulations by applying Student's T and Mann–Whitney U tests, results show that recipient's age is a characteristic that influences the quartile of the received graft. Recipients most likely to receive an organ from the quartile with the worst prognosis (i.e., the fourth) are those of more advanced age. On the other hand, days on renal replacement therapy influences to receive an organ from the third or the fourth quartile. Finally, neither the sex nor the BMI of the recipient influence the quartile to which the assigned organ belongs.
Recipient characteristics classified by KDPI quartiles of received organs.
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Age (years) | 40.48 (11.16) | 43.94 (10.95) | 50.64 (11.24) | 60.79 (9.02) |
| Gender (% male) | 228 (62.98%) | 350 (64.46%) | 451 (62.55%) | 696 (62.82%) |
| BMI (kg/m2) | 24.71 (4.65) | 26.03 (8.73) | 26.97 (9.78) | 30.38 (14.81) |
| Time on RRT (days) | 1593 (2954) | 1434 (1877) | 1866 (3722) | 1351 (1971) |
Data are presented as mean (SD) or n (%). BMI, body mass index; RRT, renal replacement therapy.
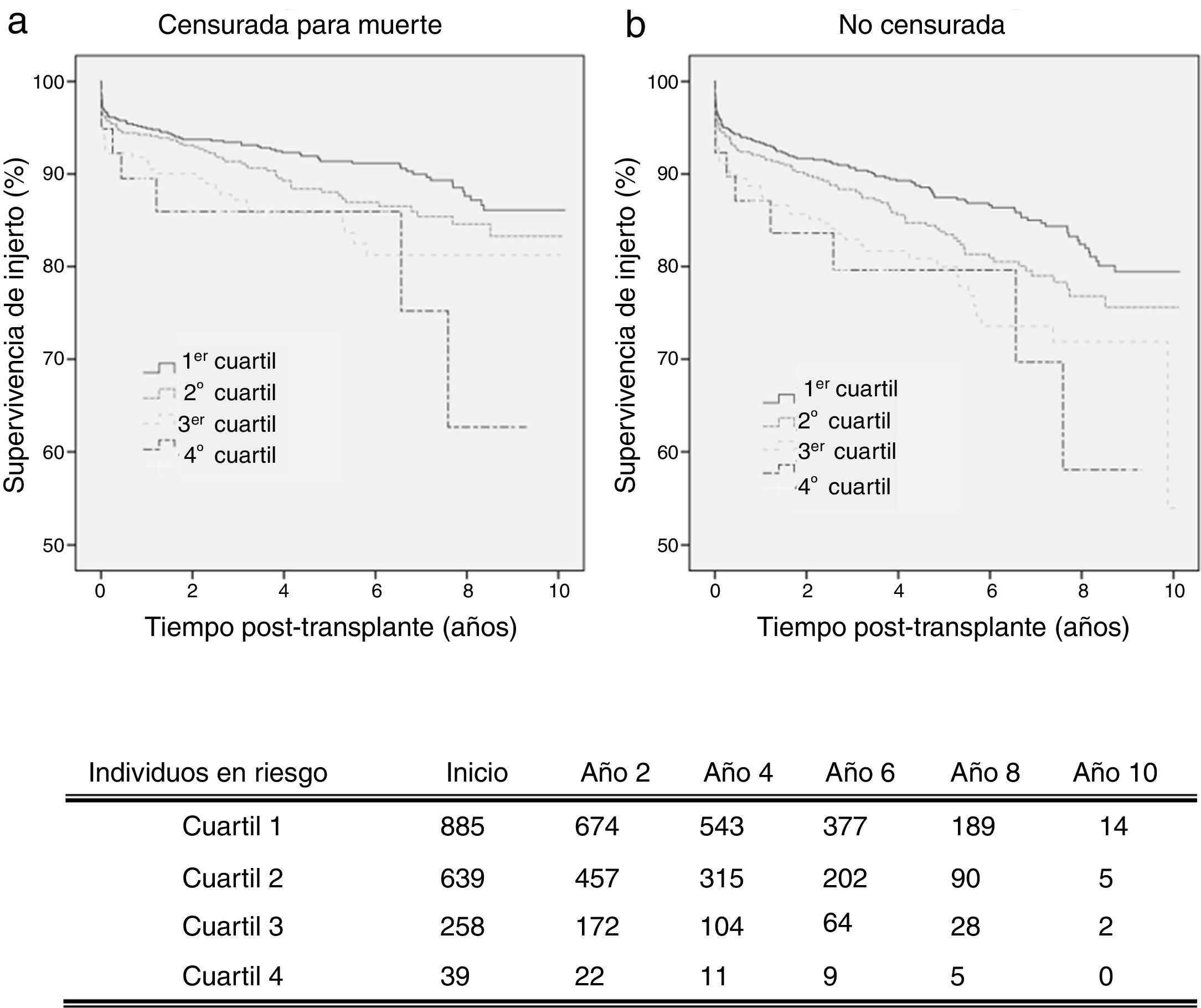
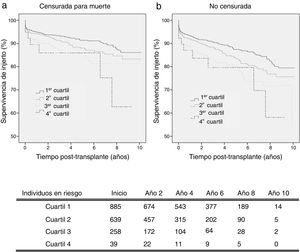
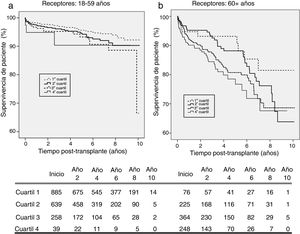
By analysing kidney graft survival by KDPI quartile for recipients of 18–59 years, it is observed that the KDPI accurately discriminate graft survival for donors with the lowest to highest risk quartiles achieving different levels of survival at 3 years (93.4%, 91.4%, 87.2%, and 85.9%) and 5 years (91.4%, 88.1%, 85.8% and 85.9%) (Fig. 2a). Fig. 2 compares death-censored and uncensored graft survival resulting in a slightly lower survival in the uncensored case across quartiles, what is coherent with the age range of this cohort.
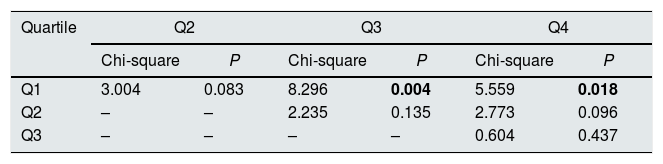
Graft survival is found significant different between the first quartile (optimal donors) and the third and fourth quartiles (marginal donors), but there is no difference between quartiles of medium and low quality (Table 3). Then, it can be stated that the KDPI, from a particular donor quality level, stops being useful to discriminate graft survivals. For this recipient group, a univariate Cox regression analysis showed that KDPI, when analysed as a continuous variable, was associated with death-censored graft survival in this group with a hazard ratio of 1.013 (P<0.001; 95% CI: 1.006–1.019).
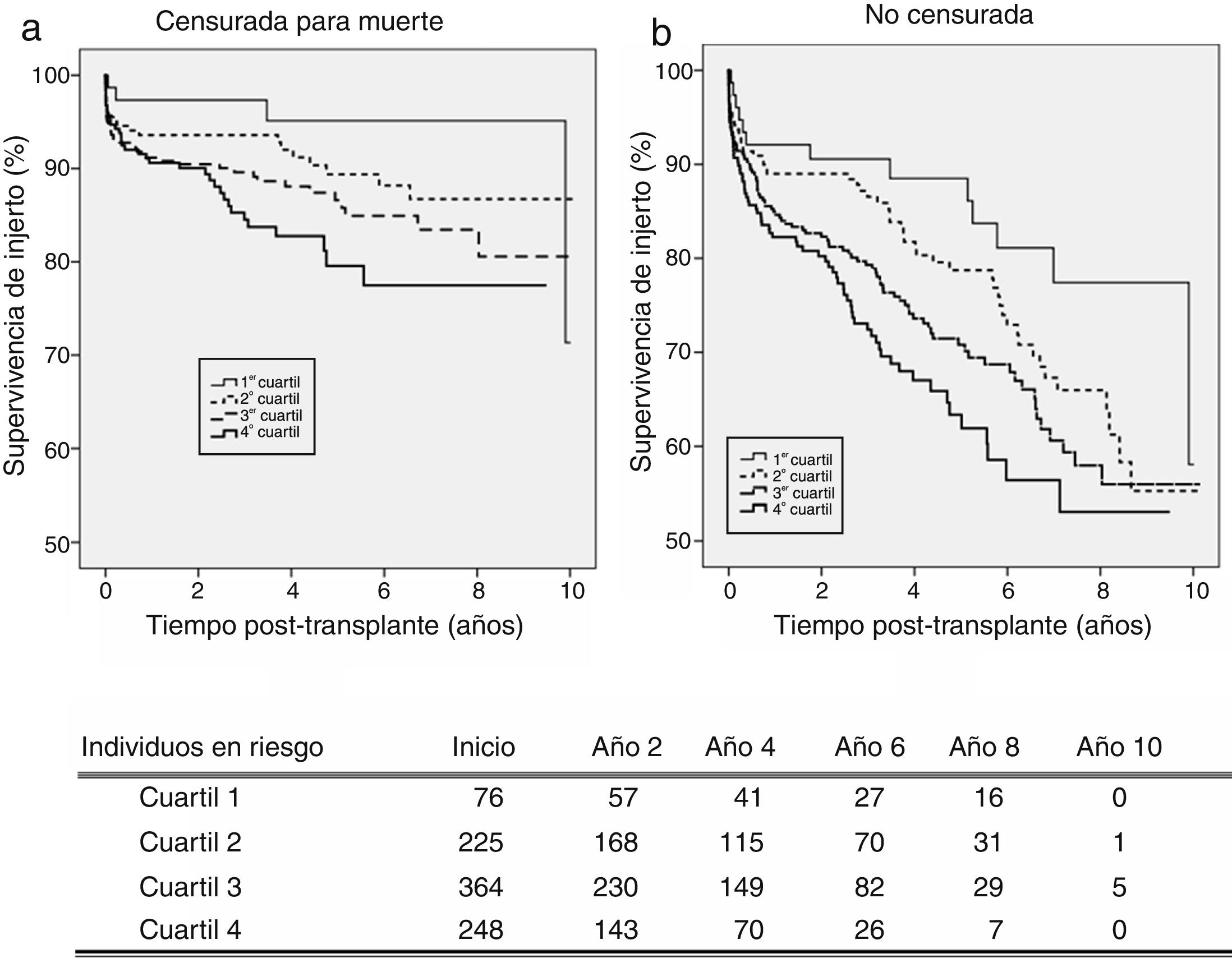
On the senior population (60+ years), kidney graft achieves 3-year survival of 97.3%, 93.6%, 89.6%, and 84.5%; and 5-year survival of 95.1%, 89.4%, 86.6%, and 79.6%, respectively (Fig. 3a). These survival levels are similar (or even higher) to those reported above for the cohort of 18–59 years since they refer to death-censored graft survival. The uncensored graft survival analysis (Fig. 3b) shows how the recipient's age leads to a significant reduction of survival for all the quartiles.
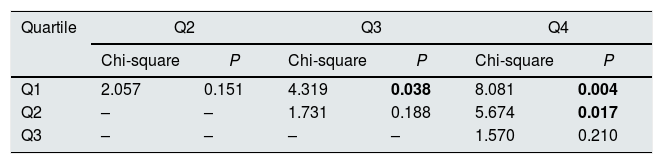
The KDPI does not discriminate between consecutive quartiles in the group of recipients older than 60 years (Table 4). As occurred on the adult population, the higher the KDPI, the worse graft survival. Moreover, the KDPI discriminates among optimal and marginal quality donors but not among medium and low-quality donors. Finally, the univariate Cox regression analysis showed that the KDPI was significantly associated with death-censored graft outcome with a hazard ratio of 1.016 (P=0.001; 95% CI: 1.006–1.025).
Finally, we analysed the discriminatory ability of the KDPI in Spain by means of Harrell's C. For a binary outcome (graft survival vs. death-censored graft loss), a C index of 0.5 represents a prediction no more accurate than chance. Across all quartiles and for the whole population (age: 18+ years), the Harrell's C of the KDPI for graft and patient survival was 0.56 and 0.63, respectively. This is a predictive power limited and equivalent to the results in the USA (i.e., C-statistic=0.62).2
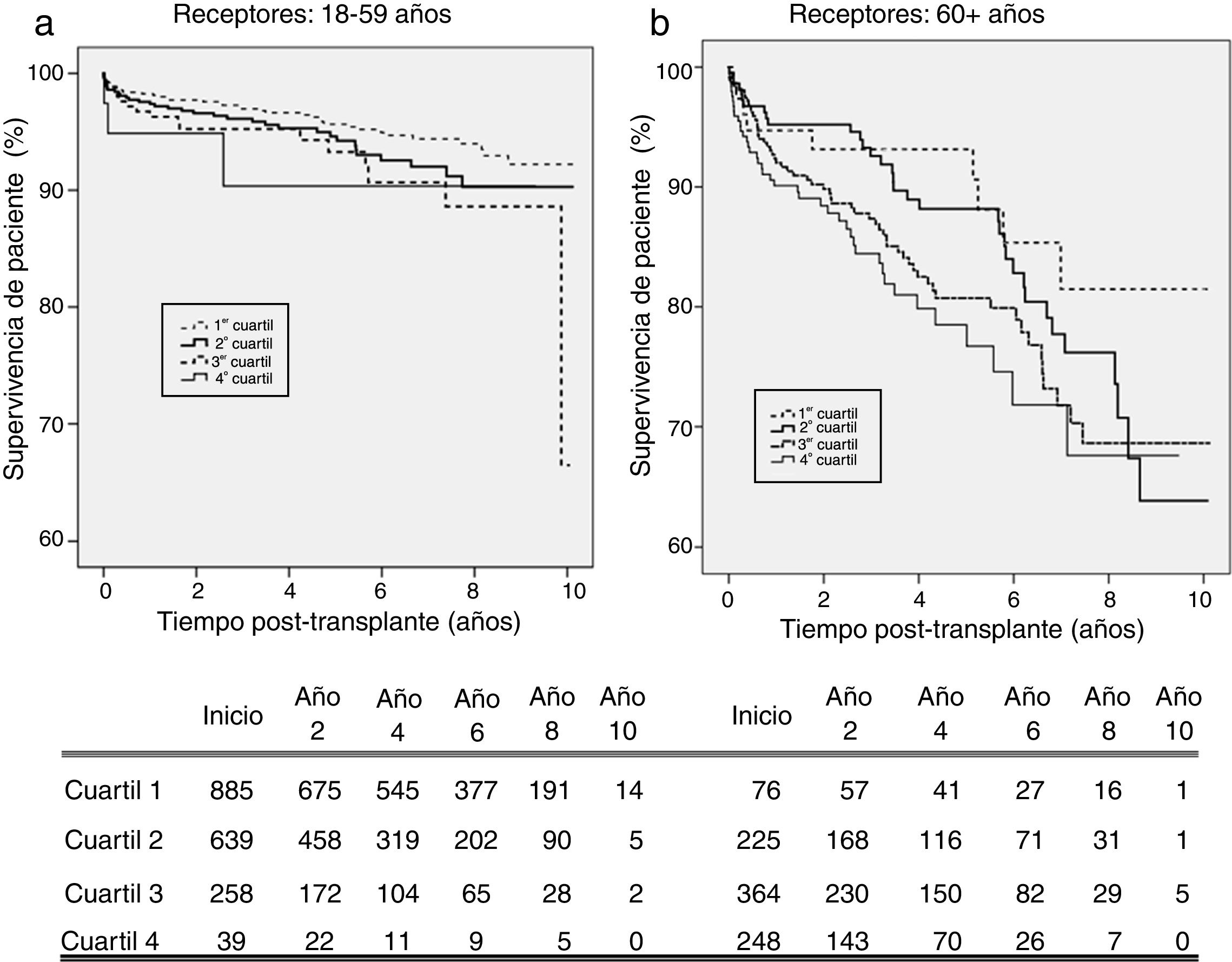
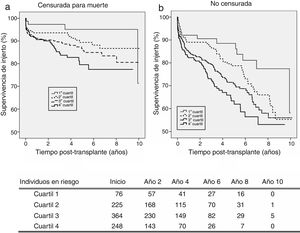
KDPI and patient survivalFig. 4 shows patient survival for recipients of 18–59 years (a) and 60+ years (b). For the first subpopulation, KDPI shows minor discrimination among quartiles, although the hazard ratio of patient survival associated to KDPI for this group is 1.013 (P=0.007; 95% CI: 1.004–1.023). For recipients older than 60 years, KDPI shows major differences among quartiles (Fig. 4b), and the hazard ratio for patient survival is 1.011 (P=0.007; 95% CI: 1.003–1.018). Therefore, KDPI is a potential indicator of patient survival although with a lower discrimination between quartiles.
Multivariate analysisA set of candidate predictors (i.e., a priori predict graft survival) were selected based on the literature.15–17 A univariate Cox regression analysis was performed for each predictor to assess its relevance on graft survival. The following predictors related to recipient and transplant factors were significant: age, gender, height, HIV status, HCV status, primary renal disease, HLA mismatch, and time on renal replacement therapy. Donor factors were included in two separate ways: as part of the KDPI and as individual factors. Two Cox proportional hazards graft survival analysis were performed (Table 5).
Significant factors in a risk-adjusted Cox proportional hazards graft survival analysis. Adult deceased donor and adult recipient performed in Spain, January 1, 2006, to December 31, 2015.
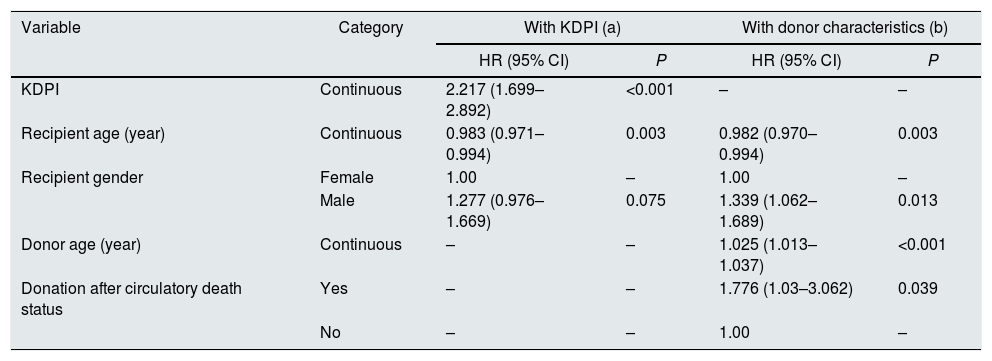
| Variable | Category | With KDPI (a) | With donor characteristics (b) | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| KDPI | Continuous | 2.217 (1.699–2.892) | <0.001 | – | – |
| Recipient age (year) | Continuous | 0.983 (0.971–0.994) | 0.003 | 0.982 (0.970–0.994) | 0.003 |
| Recipient gender | Female | 1.00 | – | 1.00 | – |
| Male | 1.277 (0.976–1.669) | 0.075 | 1.339 (1.062–1.689) | 0.013 | |
| Donor age (year) | Continuous | – | – | 1.025 (1.013–1.037) | <0.001 |
| Donation after circulatory death status | Yes | – | – | 1.776 (1.03–3.062) | 0.039 |
| No | – | – | 1.00 | – | |
(a) Hazard ratios are adjusted for factors in the table, and recipient height, HIV and HCV status, primary renal disease, HLA mismatch, time on RRT.
(b) Hazard ratios are adjusted for factors referred in (a) and donor factors: gender, hypertension, creatinine level, height, weight, history of diabetes and hepatitis, and cause of death.
HR, hazard ratio; CI, confidence interval; HLA, human leucocyte antigen.
In the first analysis (i.e., donor factors included as KDPI, Table 5a), the KDPI was the most significant factor predicting graft survival. Recipient age and gender are also significantly related to the transplant outcome. The hazard ratio of recipient age is lower than 1 (0.983) what is coherent with the type of analysis performed, death-censored. Censoring deaths converts recipient age in a protecting factor of graft loss, that is consistent with several studies.18
When KDPI donor factors are analysed separately (Table 5b), donor age and donation after circulatory death status are the predicting factors of graft survival (in addition to recipient age and gender). There was no evidence of prediction by the other factors with the study cohort.
DiscussionThe KDPI may mean a significant improvement for decision-making, but its discriminatory power on graft survival is limited (Harrell's C, 0.56) and the feasibility of high-KDPI grafts (sometimes discarded) on recipients older than 60 years is uncertain. Results obtained are in accordance with those of a recent study validating donor-only KDRI (i.e., KDPI) on a Dutch population and obtaining a discriminatory ability (Harrell's C) of 0.62.19
Our work states that KDPI discriminates graft and patient survival for recipients of 18–59 years, with differences between recipients of optimal donors (KDPI<25%) and the rest. For recipients older than 60 years, the KDPI prediction power is equally limited, but even then, it rightly discriminates between the first, the third and the fourth quartiles (i.e., between optimal and marginal donors). The KDPI is also useful for discriminating patient survival among quartiles. It is noteworthy in both subpopulations the poor discrimination above the median (third and fourth quartiles); this might suggest a lack of accuracy of the index from a certain level of quality, or that others characteristics not collected by the index (e.g., biopsy) are involved.
Regarding recipients older than 60 years, there is an association between graft survival and KDPI. Several studies have analysed different effects caused by expanded or high-KDPI donors according the recipient's age, stating that the influence on survival is lower in older recipients. Ma et al. studied if the use of SCD or ECD kidneys influences differently on patient survival if the recipient's age is higher or lower than 60 years.20 They analysed 3822 cases between 1997 and 2009 stating that in younger recipients there was an excess risk of all-cause mortality (adjusted HR=1.55; 95% CI: 1.23–1.97) and death with functioning graft (adjusted HR=1.72; 95% CI: 1.28–2.29) after transplantation with ECD kidneys compared with SCD kidneys. This conclusion could not be applied to older recipients (adjusted HR=1.11; 95% CI: 0.80–1.54, and adjusted HR=1.30; 95% CI: 0.89–1.89, respectively). The authors set that an excess risk of all-cause mortality on younger recipients of an ECD kidney is caused by the increase of cardiovascular mortality associated to worse renal function of the graft (and reduced GFR) obtained with this kind of donor. Chronic inflammation and the state of immunosuppression associated with uraemia lead to accelerated atherosclerosis that would justify these poor results.21 Our work agrees since the survival of younger recipients is clearly affected by the KDPI (Fig. 4a). It also strengths the importance of maintaining the principle of allocation ‘old for old’.
Hernandez et al. studied the odds of death and graft loss regarding organ quality measured by KDPI, divided in five categories, among cohorts of recipients with different age ranges.22 The survival of recipients from 50 to 69 years receiving a low-quality graft is significantly reduced. However, recipients from 70 to 79 years receiving a very low-quality graft had a lower death risk than recipients from 50 to 69 years. Recipients older than 79 years had the lowest risk. These results are consistent with our study since the KDPI hazard ratio for patient survival is lower in the population over 60 years (HR=1.011) than among those aged 18–59 years (HR=1.013).
A major objective of the US allocation system is to allocate low-KDPI grafts (better quality) to patients with high estimated survival (based on the Estimated Post Transplant Survival, EPTS7). Thus, young recipients benefit from the best-quality donors. When a high-KDPI graft is allocated to a senior recipient, the deleterious effect on patient survival is manifest. However, our results state that recipients older than 60 years can also benefit of extended survival (as Fig. 4b shows). Although allocation systems in Spain and US are different, motivation is alike. The aim is to obtain a longevity matching, so cases of allocation of optimal donors to older recipients are uncommon.
Finally, there is a couple of potential limitations of this work. Firstly, KDPI is calculated by using the study population itself. It would be more appropriate to use a reference European donor population (as it is done in US) but there is no such registry available. The authors think that it is better to use the study cohort itself to calculate the KDPI than using the completely different population published by the US Organ Procurement and Transplantation Network.23 Secondly, for the retrospective registry analysis performed, some cases had to be excluded due to missing data. Missing data is due to random failure in manual registry on data introduction, so there is no evidence to suggest this has a negative effect on interpretation. Therefore, the analysis performed are so-called complete-case analysis, and no data imputation technique has been used to replace missing data.
In summary, this is one of the first studies to validate the use of KDPI in a Spanish cohort. However, due to certain limitations on the KDPI translation to Spain, creating a new index from Spanish or European data would be advisable.24 In this work, some predictive factors of graft survival are identified that can server as a first step in this path.
Although it is correlated significanty with the graft and patients’ survival, more studies should be performed aiming to increase the predictive power of kidney donor scores or complement them with other data sources that mean a real clinical aid to professionals in pretransplantation. Increasingly, recipients demand wider information about the graft quality to receive, and the KDPI is a right step in that direction. However, new technology trends may bring tools that integrate information from different data sources (clinical, biological, histological, …) about donors and recipients to perform survival estimations with higher predictive power.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work has been partially supported by the Biomedical Engineering Group at University of Sevilla, and a grant from the Fondo de Investigación Sanitaria inside project PI15/00306.
Please cite this article as: Calvillo-Arbizu J, Pérez-Valdivia MA, Gentil-Govantes MA, Castro-de-la-Nuez P, Mazuecos-Blanca A, Rodríguez-Benot A, et al. ¿Predice el Kidney Donor Profile Index (KDPI) la supervivencia del injerto y del paciente en una población española?. Nefrologia. 2018;38:587–595.