The renoprotective effect of renin-angiotensin (RAS) blockers (angiotensin converting enzyme inhibitors and angiotensin receptor blockers) has been questioned in patients with advanced chronic kidney disease (CKD). Moreover, combination therapy (dual RAS blockade) can further accelerate renal function decline in some populations at risk. However, it is unknown whether this adverse outcome is due to a dose-dependent effect or if it can be attributed more specifically to a drug interaction.
AimThis study aims to investigate if the rate of renal function decline in advanced CKD patients is associated to the doses of RAS blockers, and if dual RAS blockade worsens renal function independently of major confounding factors.
Material and methodsRetrospective, observational study in an incident cohort of adult patients with CKD stage 4 or 5 not on dialysis, treated with RAS blockers for at least 6 months prior to the study inclusion. Inclusion criteria were: having at least three consecutive measurements of estimated glomerular filtration rate (eGFR) in a follow-up period >3 months. Decline in renal function was estimated as the slope of the individual linear regression line of eGFR over follow-up time. Equipotent doses of RAS blockers were normalised for a body weight of 70 kg or a body surface area of 1.73 m2 (END-RASI). Associations of END-RASI or dual RAS blockade with the rate of renal function decline were analysed by uni- or multivariate linear regression models, accounting for major confounding variables.
ResultsThe study group consisted of 813 patients (mean age 64 ± 14 years, 430 males) with a mean eGFR 14.9 ± 4.2 ml/min/1.73 m2. 729 patients were on RAS blockade monotherapy and 84 on dual RAS blockade. Median END-RASI in the whole group was 0.91 (I.Q. ranges 0.69–1.20). Patients on dual RAS blockade had significantly higher END-RASI than the rest of study patients (1.52 ± 0.49 vs. 0.93 ± 0.44; p < 0.0001). In univariate linear regression, END-RASI were significantly correlated with eGFR decline (R = −0.149; p < 0.0001). Patients on dual RAS blockade showed a significantly faster decline of renal function than the rest of the study patients (−6.19 ± 5.57 vs. −3.04 ± 5.37 ml/min/1.73 m2/year, p < 0.0001). By multivariate linear regression, while dual RAS blockade remained independent and significantly associated with faster renal function decline (beta = −0.094; p = 0.005), END-RASI (normalised either for body weight or surface area) did not reach statistical significance.
ConclusionEND-RASI are significantly associated with the rate of renal function decline in advanced CKD patients. However, the detrimental effect of dual RAS blockade on CKD progression seems to be independent of END-RASI and other major confounding factors.
El efecto reno-protector de los fármacos inhibidores del sistema renina-angiotensina (ISRA) ha sido cuestionado en la enfermedad renal crónica (ERC) avanzada. La combinación de tratamiento ISRA (doble bloqueo) puede, además, acelerar el deterioro de la función renal en algunas poblaciones de riesgo. Sin embargo, se desconoce si este efecto adverso está relacionado con la dosis total prescrita de ISRA o más específicamente con una interacción farmacológica.
ObjetivoInvestigar si la tasa de reducción de función renal en la ERC avanzada se asocia a la dosis total de ISRA, y si el doble bloqueo SRA deteriora la función renal independientemente de los principales factores de confusión.
Material y métodosEstudio retrospectivo de observación en una cohorte de pacientes adultos con ERC estadio 4–5 pre-diálisis, tratados con ISRA desde al menos 3 meses antes de la inclusión en el estudio. Otros criterios de inclusión fueron: tener al menos 3 medidas consecutivas de filtrado glomerular durante un periodo superior a 3 meses. Las dosis equipotentes de ISRA fueron normalizadas (DEN-ISRA) a un peso corporal de 70 kg o una superficie corporal de 1,73 m2. La asociación de DEN-ISRA o doble bloqueo con la progresión ERC fue analizada mediante modelos de regresión lineal uni- y multivariante, tomando en cuenta las principales variables de confusión.
ResultadosSe incluyeron 813 pacientes (edad media 64 ± 14 años, 430 hombres) con un filtrado glomerular medio 14,9 ± 4,2 ml/min/1,73 m2; 729 pacientes eran tratados con ISRA monoterapia y 84 pacientes con doble bloqueo. La mediana de la DEN-ISRA en el grupo total de estudio fue de 0,91 (rangos I.Q. 0,69–1,20). Los pacientes con doble bloqueo tenían una DEN-ISRA significativamente mayor que el resto (1,52 ± 0,49 vs. 0,93 ± 0,44; p < 0,0001). Mediante regresión lineal univariable, DEN-ISRA se correlacionó significativamente con la tasa de progresión de la ERC (R = −0,149; p < 0,0001). Los pacientes con doble bloqueo mostraron un deterioro más acelerado de la función renal que el resto (−6,19 ± 5,57 vs. −3,04 ± 5,37 ml/min/1,73 m2/año, p < 0,0001). Mediante regresión lineal multivariante, el tratamiento con doble bloqueo SRA mantuvo la asociación significativa e independiente con el deterioro de función renal (beta = −0,094; p = 0,005), mientras que la DEN-ISRA no alcanzó significación estadística.
ConclusiónLa DEN-ISRA se asocia de forma significativa con la tasa de progresión en pacientes con ERC avanzada. Sin embargo, el efecto negativo del doble bloqueo SRA sobre la progresión de la ERC parece independiente de la DEN-ISRA y de otros factores relevantes de confusión.
During the last decades, renin-angiotensin system Inhibitors (RAS inhibitors ) have been the main therapeutic resource to slow down the progression of chronic kidney disease (CKD) of various etiologies.1–4 Both angiotensin conversion enzyme (ACEI) inhibitors and angiotensin II receptor antagonists (ARA), in addition to the effect on blood pressure control, they have also ascribed other benefits: hemodynamic, antiproteinuric, pleiotropic (antifibrogenic, etc.) that could justify the qualification of "reno-protectors".1–5
These favorable effects on the progression of CKD have been demonstrated based on rigorous studies in experimental animals and numerous clinical trials, well summarized in the review by Čertíková Chábová and Červenka.6 Such results strongly supported the strict control of the renin-angiotensin system (RAS) to the point that during the first decade of this century it was proposed to maximize the doses of these drugs and even combine them (dual RAS blockade with ACEI plus ARB), pursuing therapeutic objectives beyond the strict control of blood pressure, such as the reduction of proteinuria.5
High doses of RAS inhibitors and even dual blockade have been shown to be beneficial in the evolution and survival of some cardiovascular diseases,3,7–9 however the frequent association with accelerated decline of renal function and development of other adverse effects have questioned this treatment strategy in CKD, and especially in some kidney diseases such as diabetic nephropathy, or in patients with advanced CKD.6,10–14 Therefore there is a number of authors with opinions against the unrestricted use of these drugs in CKD.15–22 generating a clinical-therapeutic controversy that deserves to be investigated.
We have observed that in patients with advanced CKD the dual RAS blockade is associated with a faster decline of kidney function.23 Another important point of these previous observations is the great heterogeneity in the prescribed doses of RAS inhibitors adjusted to the patient weight or body surface. Thus, we were motivated to formulate the following questions: is there an association between the total adjusted dose of ISRA and the progression of advanced CKD?, and could the negative effect of the double RAS blockade on the progression of CKD be justified by a high total dose of RAS inhibitors or is it independent of the dose and other important confounding factors?
With the objective of answering these questions, the present observational study was performed in a cohort of patients with advanced CKD on treatment with RAS inhibitors.
MethodsRetrospective longitudinal observational study in a cohort of adult patients diagnosed with CKD stages 4–5 not on dialysis, followed in the advanced CKD (ACKD) outpatient clinic from January 2000 to December 2016. To be included in the study patients had to be followed in the ACKD clinic for more than 3 months; with at least 3 measurements of renal function; and being treated with RAS inhibitors for a minimum of 3 months before being included in the study.
All patients were referred to the ACKD clinic for progressive decline of renal function. Demographic and clinical data, and prescribed medication were obtained from medical records, physical examination and anamnesis were recorded. The comorbidity was evaluated at the time of inclusion, using the Davies index,24 and patients were categorized into 3 groups: no comorbidity, mild-moderate, or severe comorbidity.
Biochemical tests were performed in the same central laboratory (Clinical Analysis Service of Infanta Cristina Hospital) using conventional methods (Advia Chemistry Autoanalyzer, Siemens Healthcare Diagnostics, New York, USA ), in fresh samples ( not stored), and both the calibrations and the traceability of creatinine were performed according to the recommendations of international NKDEP standards.25 Glomerular filtration was estimated using the abbreviated formula MDRD.26
Patients were followed regularly with visits every 30–90 days. The rate of kidney function decline in each patient was determined by the linear regression of the estimated glomerular filtration rate over time elapsed between each control and the first appointment, with an accuracy of days. The resulting slope of this linear equation was expressed in ± ml / min / 1.73 m 2 / year. The negative or positive values of this slope meant progression of renal insufficiency or recovery of renal function, respectively.
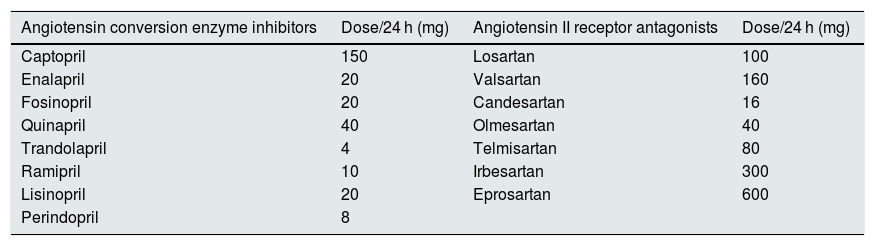
The assessment of the comparative power of RAS inhibitors (equipotent dose) of each drug used was estimated following the prescription recommendations of the manufacturing laboratories on the daily doses required for blood pressure control in adults. This information is collected in bulletins of the Spanish Agency for Medicines and Health Products (AEMPS) as detailed in the Table 1.
Equipotent daily dose of the renin-angiotensin system inhibitors used in this study. Each of these doses was taken as a therapeutic unit before adjusting to weight or body surface.
| Angiotensin conversion enzyme inhibitors | Dose/24 h (mg) | Angiotensin II receptor antagonists | Dose/24 h (mg) |
|---|---|---|---|
| Captopril | 150 | Losartan | 100 |
| Enalapril | 20 | Valsartan | 160 |
| Fosinopril | 20 | Candesartan | 16 |
| Quinapril | 40 | Olmesartan | 40 |
| Trandolapril | 4 | Telmisartan | 80 |
| Ramipril | 10 | Irbesartan | 300 |
| Lisinopril | 20 | Eprosartan | 600 |
| Perindopril | 8 |
Each of these daily doses was considered a therapeutic unit of similar potency (equipotent), which was added to combined treatments, and was normalized to a body weight of 70 kg or a body surface area of 1.73 m 2, thus a standardized equipotent dose parameter (SED) was established. For example, a patient with a body weight of 70 kg who took 20 mg of enalapril or 160 mg of valsartan or 16 mg of candesartan daily had a SED of 1. If these same doses were prescribed to a patient weighing 100 kg, the resulting SED would be 0.7; and if they were prescribed to a patient of 50 kg, the resulting SED is 1.4. If treatments were combined (double blockade) such as 20 mg of enalapril and 16 mg of candesartan in a 70 kg patient, the resulting SED is 2, and so on.
Study design and statistical methodsThe study is a retrospective longitudinal observation.
Demographic, clinical and biochemical characteristics of patients treated with RAS inhibitors monotherapy were compared with those treated with double blockade.
The degree of correlation between the normalized doses of RAS inhibitors (SED- RAS inhibitors) and the progression of CKD was analyzed by uni- and multivariate linear correlation. In addition to the (SED- RAS inhibitors) the independent variables included in the analysis were : dual blockade of RAS, age, sex, comorbidity index, body mass index, smoking, diabetes mellitus, initial renal function (baseline), blood pressure (baseline), 24 h urine proteinuria, serum bicarbonate, and concomitant treatment with diuretics, beta blockers, and calcium channel antagonists. The automatic conditional progressive elimination process was used to select the variables for the best prediction models.
Depending on their characteristics, parametric or non-parametric tests were used for the descriptive comparison of the continuous variables; chi-square test was used to compare categorical variables.
Descriptive statistical data are presented as mean and standard deviation, or as median and interquartile ranges for continuous variables, and as percentages in the case of categorical variables. A p < 0.05 was considered statistically significant, and all p values shown are bilateral. Statistical analyzes were performed with the IBM SPSS Statistics 24.0 software (IBM Corp. Armonk, USA ).
ResultsDuring the inclusion period, a total of 1580 incident patients were treated in the ACKD clinic and 1079 patients fulfilled the follow-up time criteria. Of these, 813 met the criteria of being treated with RAS inhibitors for more than 3 months before inclusion, and these were the patients included the study.
The demographic, clinical and biochemical characteristics of the total study group are shown in Table 2.
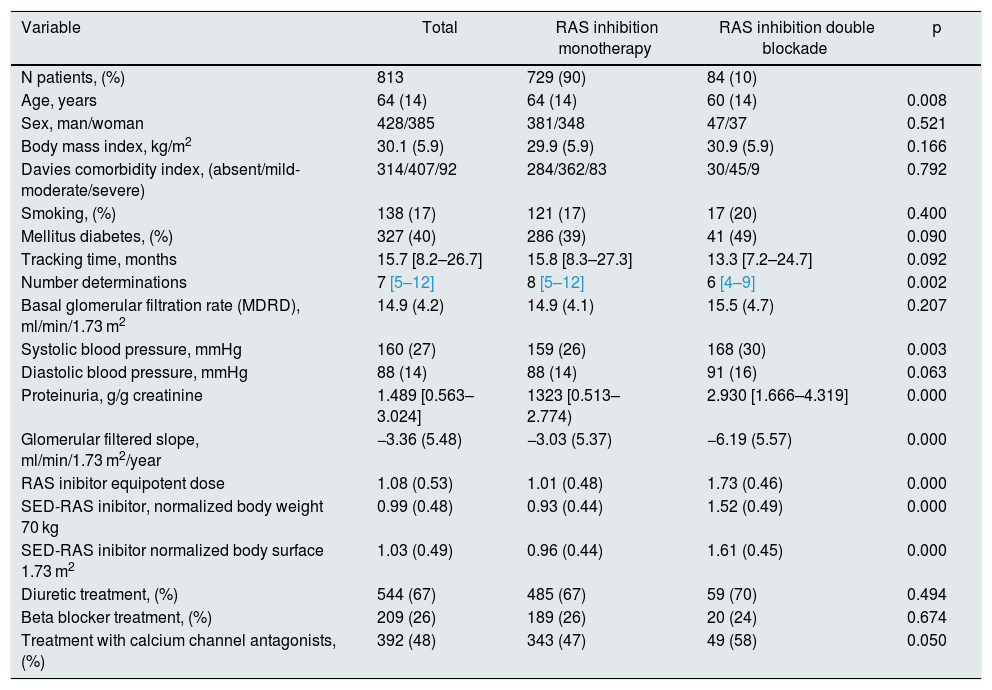
Demographic, clinical and biochemical characteristics in the total group of patients separated according to whether they were treated with RAS inhibitors alone or with double blockade.
| Variable | Total | RAS inhibition monotherapy | RAS inhibition double blockade | p |
|---|---|---|---|---|
| N patients, (%) | 813 | 729 (90) | 84 (10) | |
| Age, years | 64 (14) | 64 (14) | 60 (14) | 0.008 |
| Sex, man/woman | 428/385 | 381/348 | 47/37 | 0.521 |
| Body mass index, kg/m2 | 30.1 (5.9) | 29.9 (5.9) | 30.9 (5.9) | 0.166 |
| Davies comorbidity index, (absent/mild-moderate/severe) | 314/407/92 | 284/362/83 | 30/45/9 | 0.792 |
| Smoking, (%) | 138 (17) | 121 (17) | 17 (20) | 0.400 |
| Mellitus diabetes, (%) | 327 (40) | 286 (39) | 41 (49) | 0.090 |
| Tracking time, months | 15.7 [8.2–26.7] | 15.8 [8.3–27.3] | 13.3 [7.2–24.7] | 0.092 |
| Number determinations | 7 [5–12] | 8 [5–12] | 6 [4–9] | 0.002 |
| Basal glomerular filtration rate (MDRD), ml/min/1.73 m2 | 14.9 (4.2) | 14.9 (4.1) | 15.5 (4.7) | 0.207 |
| Systolic blood pressure, mmHg | 160 (27) | 159 (26) | 168 (30) | 0.003 |
| Diastolic blood pressure, mmHg | 88 (14) | 88 (14) | 91 (16) | 0.063 |
| Proteinuria, g/g creatinine | 1.489 [0.563–3.024] | 1323 [0.513–2.774) | 2.930 [1.666–4.319] | 0.000 |
| Glomerular filtered slope, ml/min/1.73 m2/year | −3.36 (5.48) | −3.03 (5.37) | −6.19 (5.57) | 0.000 |
| RAS inibitor equipotent dose | 1.08 (0.53) | 1.01 (0.48) | 1.73 (0.46) | 0.000 |
| SED-RAS inibitor, normalized body weight 70 kg | 0.99 (0.48) | 0.93 (0.44) | 1.52 (0.49) | 0.000 |
| SED-RAS inibitor normalized body surface 1.73 m2 | 1.03 (0.49) | 0.96 (0.44) | 1.61 (0.45) | 0.000 |
| Diuretic treatment, (%) | 544 (67) | 485 (67) | 59 (70) | 0.494 |
| Beta blocker treatment, (%) | 209 (26) | 189 (26) | 20 (24) | 0.674 |
| Treatment with calcium channel antagonists, (%) | 392 (48) | 343 (47) | 49 (58) | 0.050 |
Standard deviations of continuous variables are shown in parentheses, except for follow-up time, number of determinations and proteinuria (median and interquartile ranges). SED- RAS inibitor: standardized equipotent dose of renin-angiotensin system inhibitor; RAS inhibitor: renin-angiotensin system inhibitor.
The mean age was 64 years, with a small predominance of men (53%) and a high prevalence of diabetes mellitus (40%). An outstanding clinical features of this group was the presence of nephropathies with proteinuria and high blood pressure.
90% of the patients received RAS-inhibitor in monotherapy, and the SED for a weight of 70 kg or a body surface area of 1.73 m2 were 0.99 and 1.03, respectively.
The rate of progression of CKD was significantly reduced in patients receiving RAS inhibitor in monotherapy as compared with those that had prescribed dual blockade of RAS inhibitor (−3.03 ± 5.37 vs. − 6.19 ± 5.57 ml / min / 1.73 m 2 / year, p < 0.0001).
Between these 2 subgroups there were also some differences in clinical characteristics, such as younger age, higher values of systolic blood pressure, and higher proteinuria in the subgroup treated with dual blockade of RAS inhibitor as compared with those treated with monotherapy (Table 2).
As expected, the SED-RAS inhibitor of the subgroup treated with dual blockade was significantly higher than that of those treated with monotherapy (1.52 ± 0.49 vs. 0.93 ± 0.44; p < 0.0001).
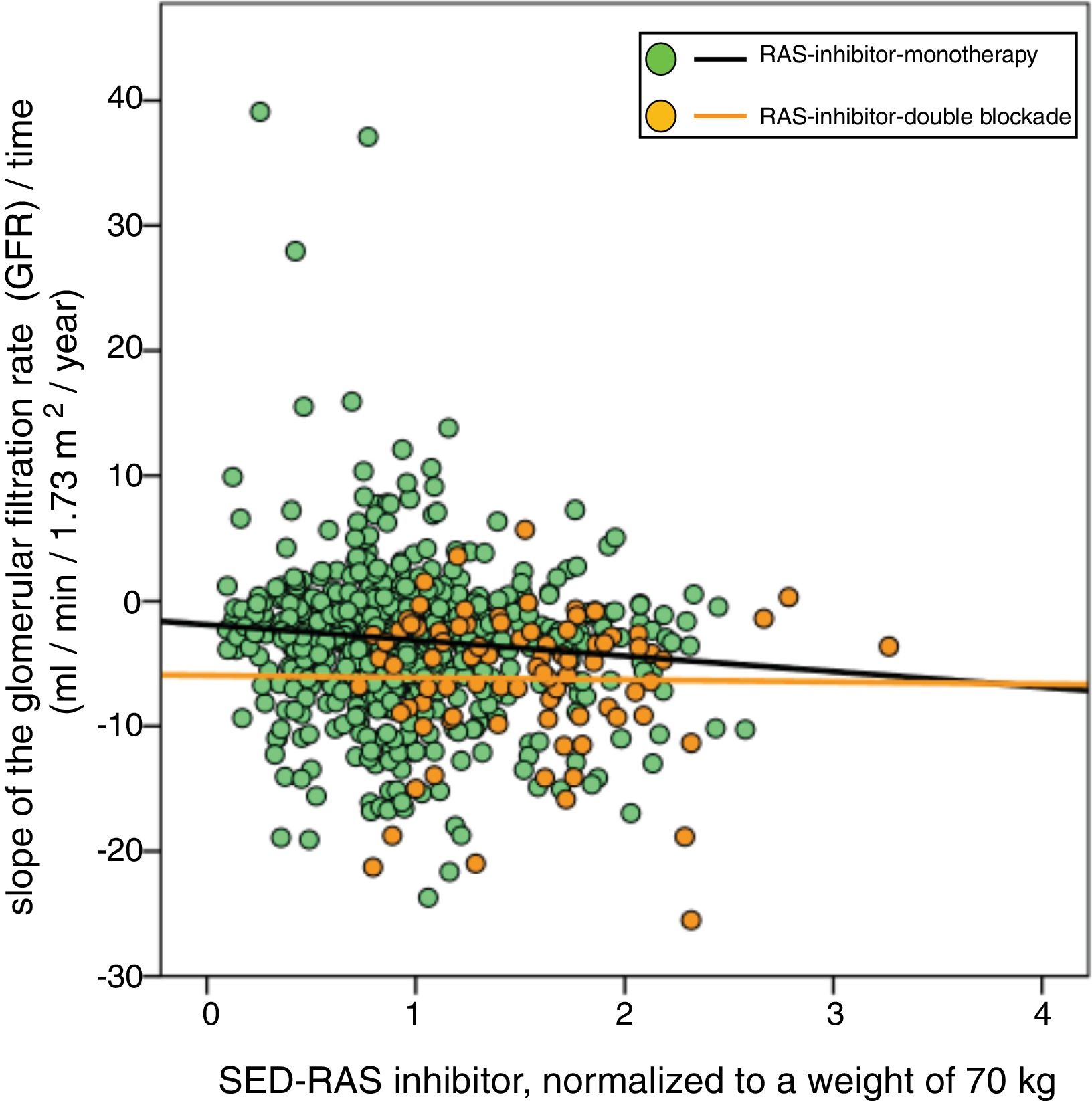
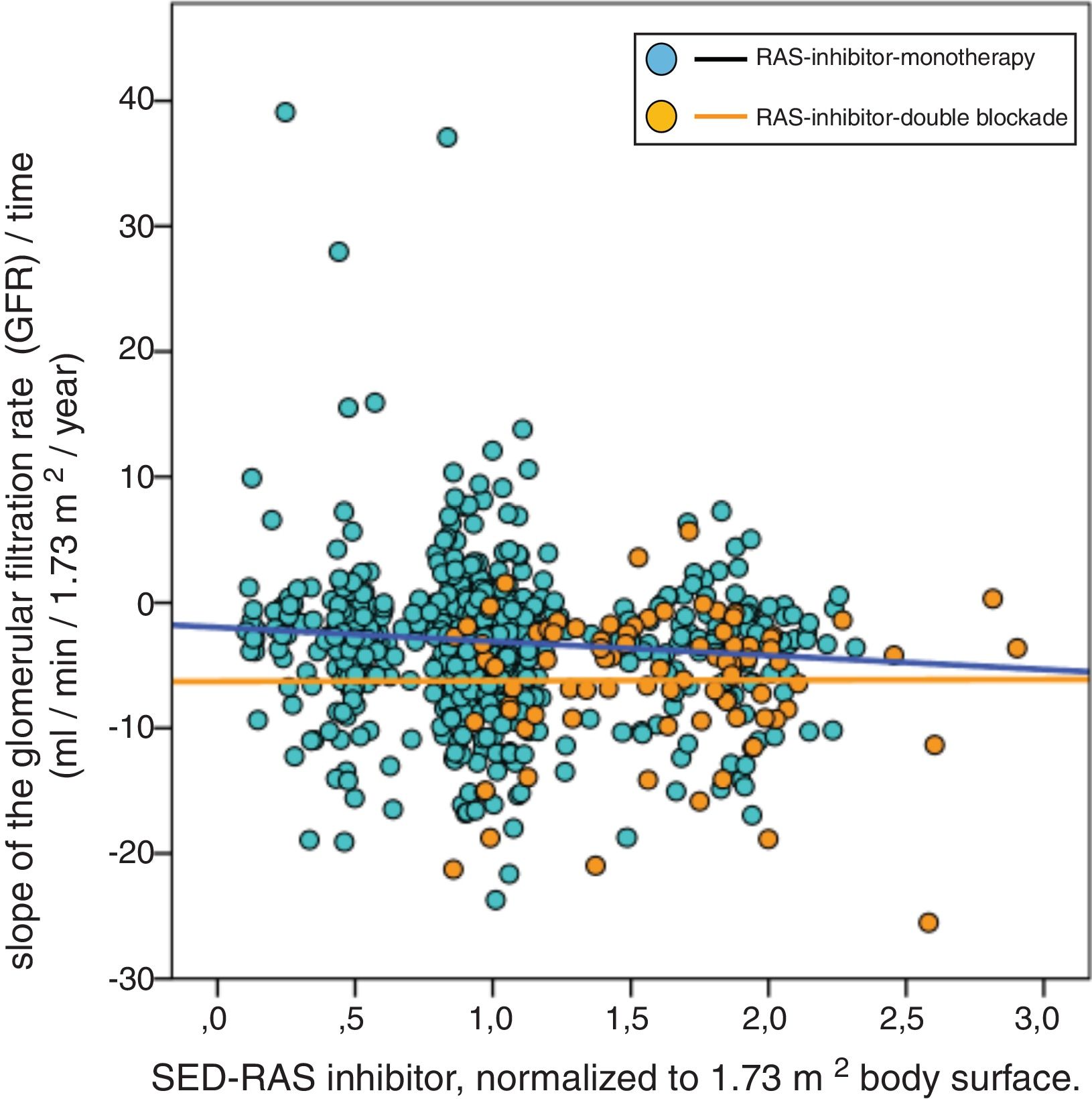
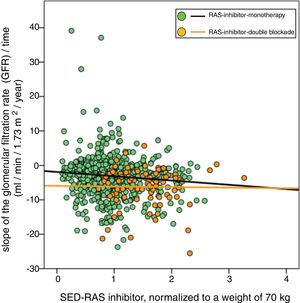
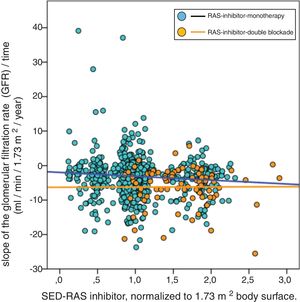
A significant linear correlation was observed between SED-RAS inhibitor and the slope GFR/ time (progression of CKD), whether normalization was performed for body weight (Fig. 1) or body surface (Fig. 2).
Linear regression between the slope of the glomerular filtration rate (GFR)/time and the standardized equipotent dose (SED) of renin-angiotensin system inhibitors (RAS-inhibitor) adjusted to a weight of 70 kg. The regression lines shown in the figure are those corresponding to the subgroups with double blockade and monotherapy, respectively.
The combined linear regression equation is: Slope GFR (ml/min/1.73 m2/year) = −1.672 – (1.71 × SED-RAS inhibitor); R = −0.149; p < 0.0001.
For monotherapy patients: Slope GFR/time = −1.88 – (1.24 × SED-RAS inhibitor); R = 0.101; p = 0.006.
For patients with double blockade: Slope GFR/time = −5.93 – (0.17 × SED-RAS inhibitor); R = 0.015; p = 0.89.
Linear regression between the slope of the glomerular filtered ratio (GFR)/time and the standardized equipotent dose (SED) of renin-angiotensin system inhibitors (RAS-inhibitor) adjusted to 1.73 m2 body surface. The regression lines that appear in the figure are those corresponding to each subgroup with double blockade and monotherapy, respectively.
The combined linear regression equation is: Slope GFR (ml/min/1.73 m2/year) = −1.685 – (1.659 × SED-RAS inhibitor); R = −0.145; p < 0.0001.
For monotherapy patients: Slope GFR/time = −1.959 – (1.120 × SED-RAS inhibitor); R = 0.093; p = 0.012.
For patients with double blockade: Slope GFR/time = −6.279 – (0.053 × SED-RAS inhibitor); R = 0.004; p = 0.969.
It was also observed that at the same SED-RAS inhibitor, patients treated with dual blockade had a more rapid deterioration of GFR (Fig. 1 and 2).
In the multivariate linear regression analysis of the variables associated with the progression of CKD, dual RAS blockade remained independently and significantly associated with the outcome variable (slope glomerular filtered rate / time), however the SED-RAS inhibitor lost statistical significance when this relationship was adjusted to the rest of the independent variables (Table 3).
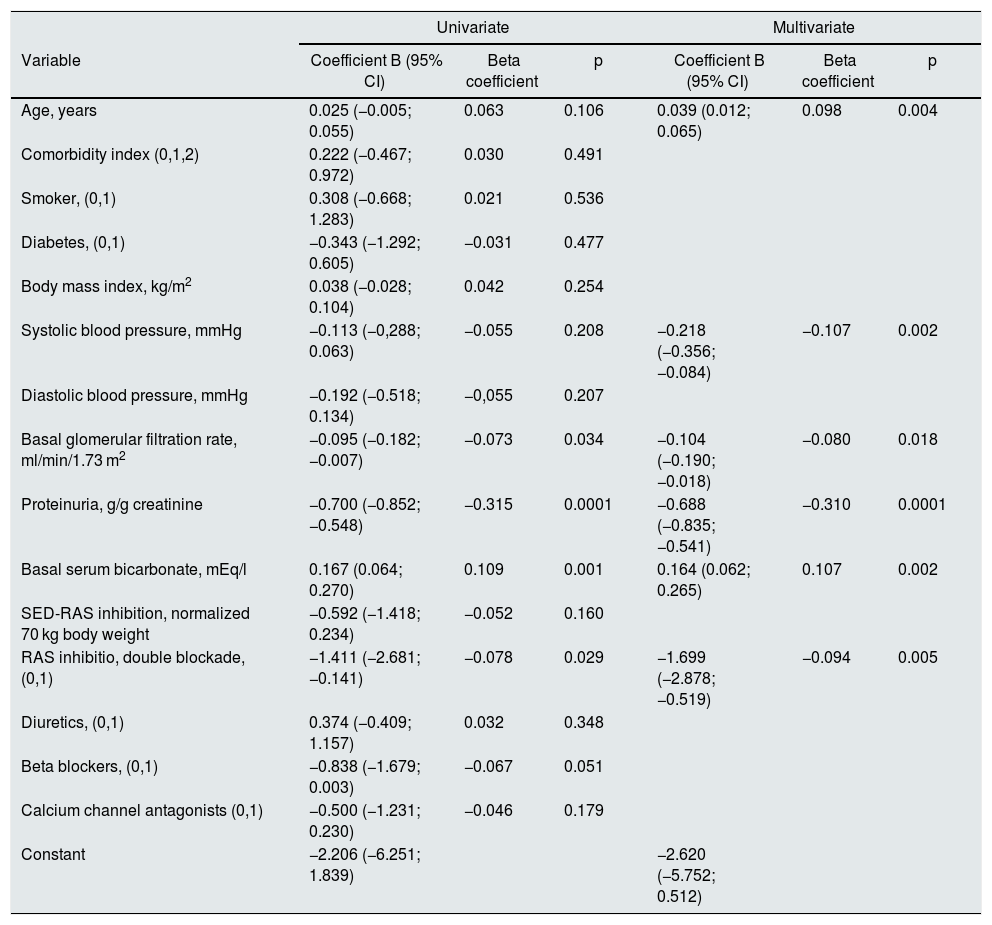
Multivariate linear regression analysis of factors associated with the progression of chronic kidney disease (slope of glomerular filtration rate/time).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient B (95% CI) | Beta coefficient | p | Coefficient B (95% CI) | Beta coefficient | p |
| Age, years | 0.025 (−0.005; 0.055) | 0.063 | 0.106 | 0.039 (0.012; 0.065) | 0.098 | 0.004 |
| Comorbidity index (0,1,2) | 0.222 (−0.467; 0.972) | 0.030 | 0.491 | |||
| Smoker, (0,1) | 0.308 (−0.668; 1.283) | 0.021 | 0.536 | |||
| Diabetes, (0,1) | −0.343 (−1.292; 0.605) | −0.031 | 0.477 | |||
| Body mass index, kg/m2 | 0.038 (−0.028; 0.104) | 0.042 | 0.254 | |||
| Systolic blood pressure, mmHg | −0.113 (−0,288; 0.063) | −0.055 | 0.208 | −0.218 (−0.356; −0.084) | −0.107 | 0.002 |
| Diastolic blood pressure, mmHg | −0.192 (−0.518; 0.134) | −0,055 | 0.207 | |||
| Basal glomerular filtration rate, ml/min/1.73 m2 | −0.095 (−0.182; −0.007) | −0.073 | 0.034 | −0.104 (−0.190; −0.018) | −0.080 | 0.018 |
| Proteinuria, g/g creatinine | −0.700 (−0.852; −0.548) | −0.315 | 0.0001 | −0.688 (−0.835; −0.541) | −0.310 | 0.0001 |
| Basal serum bicarbonate, mEq/l | 0.167 (0.064; 0.270) | 0.109 | 0.001 | 0.164 (0.062; 0.265) | 0.107 | 0.002 |
| SED-RAS inhibition, normalized 70 kg body weight | −0.592 (−1.418; 0.234) | −0.052 | 0.160 | |||
| RAS inhibitio, double blockade, (0,1) | −1.411 (−2.681; −0.141) | −0.078 | 0.029 | −1.699 (−2.878; −0.519) | −0.094 | 0.005 |
| Diuretics, (0,1) | 0.374 (−0.409; 1.157) | 0.032 | 0.348 | |||
| Beta blockers, (0,1) | −0.838 (−1.679; 0.003) | −0.067 | 0.051 | |||
| Calcium channel antagonists (0,1) | −0.500 (−1.231; 0.230) | −0.046 | 0.179 | |||
| Constant | −2.206 (−6.251; 1.839) | −2.620 (−5.752; 0.512) | ||||
SED-RAS inhibition: standardized equipotent dose of renin-angiotensin system inhibiton; CI: confidence interval; RAS inhibition: renin-angiotensin system inhibition.
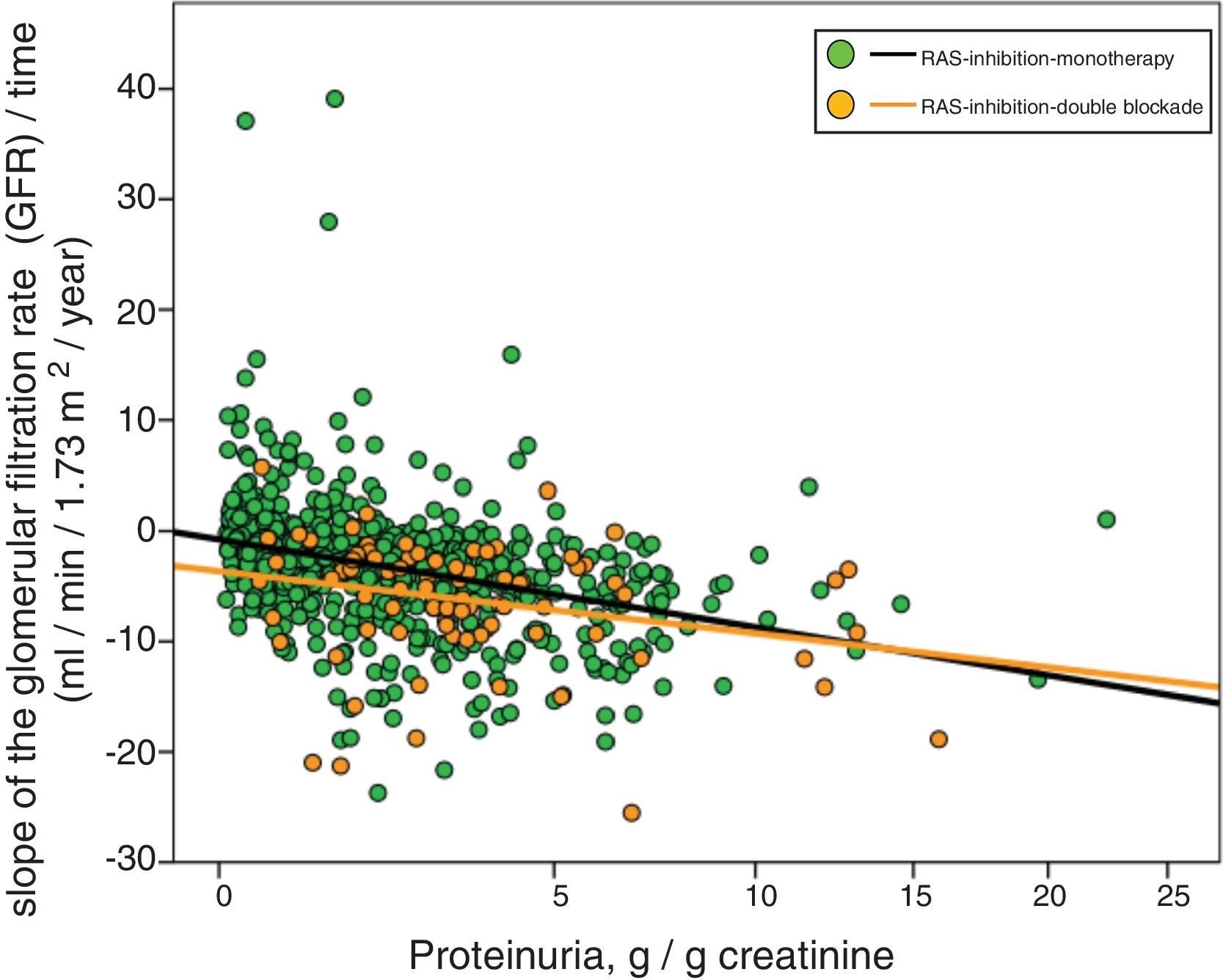
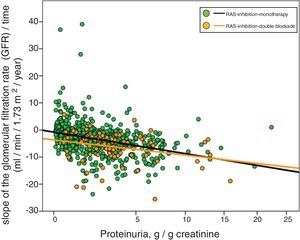
Fig. 3 shows the association between proteinuria and progression of CKD. The regression lines corresponding to the subgroup treated with monotherapy or double blockade are presented. Most evident significant differences between the subgroups were observed in patients with proteinuria less than 5 g / day.
Linear regression between the slope of the glomerular filtration rate (GFR)/time and proteinuria. The regression lines shown in the figure correspond to each subgroup, with double blockade and monotherapy, respectively. The Y axis is represented with an exponential scale 0.5.
The combined linear regression equation is: Slope GFR/time (ml/min/1.73 m2/year) = −1.720 – (0.739 × proteinuria in g/g creatinine); R = 0.332; p < 0.0001.
For monotherapy patients: Slope FG/time = −1532 – (0.727 × proteinuria in g/g creatinine); R = 0.315; p < 0.0001.
For patients with double blockade: Slope FG/time = −4409 – (0.495 × proteinuria in g/g creatinine); R = 0.275; p < 0.011.
The results of this study show that there is a significant association between the SED of RAS inhibitors and the slope of kidney failure progression in advanced stages of CKD. This progression of CKD is much faster when ACE inhibitors and ARBs (double lock) are combined, and it is independent of the SED and other relevant factors that might share the risk of progression and are a reason for prescription (for example: severity of hypertension, proteinuria, diabetes).
Although RAS inhibitors have been used to slow down the progression of CKD of various etiologies,1–4 more recently the view of some authors have questioned the use of dual blockade or even monotherapy in advanced CKD.15–22 Some observational studies and clinical trials have warned about the negative effect of dual blockade on the progression of CKD. In addition there is a clinical trial that attempts to analyze how the suspension of RAS inhibitors influences the progression of advanced CKD.27
In our patients the mean SED of RAS inhibitors approached the unit, but the wide range of prescribed doses is noteworthy. Although it was observed a significant linear regression between SED and the progression of CKD, the adjustment of the multivariate model by adding other important confounding variables ruled out a significant association between these 2 parameters. This finding uncovered the possibility of influence of the medical reason for the indication of RAS inhibitor. However, the dual blockade did maintain the independent association with CKD progression in adjusted models, including SED, as illustrated graphically in Fig. 1 and 2.
Dual blockade of RAS has demonstrated a renoprotective effect in some experimental models of renal failure in rats,6 however there are numerous clinical trials that have not been able to demonstrate this benefit in humans.10–14 This negative effect of double blockade on the progression of advanced CKD could be due to the efficacy of anti-RAS achieved with the combination of these drugs, which may be inappropriate for the pathophysiological conditions of advanced CKD such as an excessive control of systemic blood pressure and / or glomerular filtration pressure, with hemodynamic changes that may increase the risk of tubulointerstitial ischemia.
Although administration of double blockade to hypertensive rats has a very positive effect on subrogated objectives, such as blood pressure, proteinuria and left ventricular hypertrophy, paradoxically this association of drugs decreases renal function and increases glomerulosclerosis and interstitial fibrosis.28,29 It is suspected that this adverse effect is due to the inability to regulate renal blood flow as a result of "relative" hypotension and blockade of the actions of angiotensin II in the mechanism of glomerular-tubular feedback, and as a consequence of this deregulation it would cause ischemic damage and fibrosis.29
Sudden decrease in the glomerular filtration rate, up to a 30%, are frequently observed after initiation of treatment with RAS inhibitors, but it usually reverse in less than 2 months.4 In the present study, all the patients were being treated with RAS inhibitors for more than 3 months before their inclusion, therefore transitory changes in renal function can be ruled out.
The present study has limitations. Due to the retrospective design, definite causal relationships cannot be established, and since it was performed in a single center with certain treatment criteria, the results may not be generalized.
Another important limitation was the lack of control about compliance with treatment, and the effective amount of active ingredient of each prescription due to the large amount of generic medication dispensed, which as is well known and legally admitted can vary the content of active ingredient up to 20%. The pharmacokinetic peculiarities of each of the drugs used to calculate the therapeutic potency were not taken into account. Most RAS inhibitors are eliminated, in a high proportion, by the renal route, and in advanced stages of CKD a proportional dose adjustment is recommended, which is very similar for majority of RAS inhibitors.30,31
In conclusion, high SED of RAS inhibitors are associated with a more rapid deterioration of renal function in patients with advanced CKD, but this relationship loses significance when adjusted for other confounding variables. However, double blockade of RAS is independently associated with a more rapid deterioration of renal function that cannot be justified by higher SED.
According to these results, the most prudent recommendations in patients with advanced CKD would be to avoid double blockade of RAS and adjust the doses in monotherapy. Suspension of RAS inhibitors should be avoided until results of ongoing trials provide solid evidence; in these patients not only is the progression of CKD important, but also to maintain the potential cardioprotective effect of these drugs.9
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Caravaca-Fontán F, et al. Efecto negativo del bloqueo del sistema renina-angiotensina sobre la progresión de la enfermedad renal crónica avanzada: ¿una cuestión de ajuste de dosis? Nefrologia. 2020;40:38–45.