Chronic kidney disease (CKD) has been used as a model and source of knowledge concerning the mechanisms, clinical relevance and accelerated progression of cardiovascular (CV) calcification, as well as its consequences in clinical practice. However, we know that it is a late secondary ossification phenomenon and only circumstantial evidence is available. In this comprehensive review, we firstly describe the types of CV calcification which affect CKD patients, and we analyse how its presence is directly associated with CV events and increased mortality in these patients. We also justify the use of CV calcification assessment in regular nephrology clinical practice, because CV calcification is an important predictor of clinical outcome in these patients. Consequently, we believe that CV calcification assessment is a tool that could and should be used by nephrologists when making a decision concerning individual patients, consistent with the current trend of an ever-more-personalised therapeutic approach.
La enfermedad renal crónica (ERC) ha servido de modelo y fuente de conocimiento sobre los mecanismos, la relevancia clínica y progresión acelerada de los procesos de la calcificación cardiovascular (CV), así como de sus repercusiones en la práctica clínica, aunque se trate de un fenómeno tardío y secundario de osificación sobre el que solo disponemos de evidencias circunstanciales. En esta amplia revisión se describen primero los tipos de calcificación CV que afectan al paciente con ERC y se analiza cómo su presencia está directamente asociada a eventos CV y a un aumento de la mortalidad de estos pacientes. Asimismo, justificamos la valoración de la calcificación CV en la práctica clínica nefrológica habitual, al entender que es un predictor importante de la evolución clínica de estos pacientes, y consideramos que la valoración de las calcificaciones CV es una herramienta que puede y debe ser utilizada por el nefrólogo para la toma individualizada de decisiones terapéuticas en un momento en que se requiere cada vez más de una medicina personalizada.
It is currently accepted that chronic kidney disease (CKD) is an independent cardiovascular (CV) risk factor and that mortality rates increase exponentially as kidney function progressively deteriorates.1 Although the first associations between CKD and CV disease date back for more than 40 years, the true scope of the problem only became clear a little over than a decade ago. In this direction several initiatives, among others, by the Spanish Society of Nephrology, have been influential, such as implementation of a systematic estimation of the glomerular filtration rate2–7 enabling early detection of CKD and uncover hidden CKD in Spain.8 Moreover, other recent initiatives have made physicians from other specialties to be aware of the need to diagnose and stage the disease, and provide special care for kidney disease patients; to such extent that consensus documents involving up to 10 national societies have been created in Spain.6,9–11 The growing importance of CKD as a health issue, and especially for the speciality of Nephrology, is illustrated by the important value given to assessment of kidney function in clinical guidelines and publications from other specialities.12–16
In the context of the cardiorenal syndrome17 and with the close correlation between CKD and CV disease, the clinical relevance of CV calcification, even beyond kidney disease patients, is currently being widely debated.13,18–22 CKD has served as a model and source of knowledge about the mechanisms and clinical relevance of the presence and accelerated progression of the arteriosclerosis and vascular calcification processes and their repercussions in daily clinical practice.18,23–27 Knowledge about this condition is advancing at such a pace that it is worth pausing and refreshing this topic, connecting the most basic aspects with the clinical aspects and trying to be objective and realistic when reaching conclusions on the possible strategies for our patients’ care. Therefore, in the first part of this review we will briefly describe the types of CV calcification, associations with both high- and low-bone turnover diseases,24,28 and we will analyse how its presence is directly linked to CV events and increased mortality. The second part will discuss how CV calcification, despite of being a late and secondary phenomenon with only circumstantial evidence available,19,20 is a modifiable risk factor to which we can unfortunately contribute with unwanted iatrogenia.18,29
CKD, cardiovascular risk, and “CKD-MBD”In addition to the traditional CV risk factors (advanced age, obesity, tobacco use, diabetes, hypertension, dyslipidaemia), a set of non-traditional factors may explain the disproportionate mortality observed in the CKD population. These include a series of modifiable parameters of bone-mineral metabolism disorders such as changes in phosphorus (P), calcium (Ca), parathyroid hormone (PTH), vitamin D, or the fibroblast growth factor 23 (FGF23)/klotho axis, among others30–32; other factor directly or indirectly related include: inflammation, oxidative stress, or changes in the Wnt/β-catenin signalling pathway.19,33–36 The new widely accepted term CKD-MBD (acronym for chronic kidney disease-mineral and bone disorder, with inconsistent Spanish translation39,40) describes the systemic consequences and the organ damage beyond bone caused by altered mineral metabolism in CKD patients.37,38 The CKD-MBD term includes vascular, valve, and extraskeletal calcifications in addition to the biochemical and bone abnormalities. These vascular calcifications are also recognised from a pathophysiological perspective within the new concept of “bone-vascular axis”, which directly relates bone with the CV system. Now we are aware that the bone is an endocrine organ that secretes hormones such as FGF23, sclerostin, or osteocalcin, among others and that is essential part of CKD.41–47 This association affects not only CKD patients, but patients from other specialities.48,49
Anatomic location and histological types of cardiovascular calcificationThe presentation of CV calcification may be very heterogeneous. The following types have been described: 1) classical atherosclerosis-atheromatosis, in the context of degenerative changes of the aorta and large elastic arteries, and directly linked to inflammation and dyslipidaemia19,50; 2) medial calcification or Mönckeberg's disease, in the context of concentric thickening of the muscular layer media of the arteries50; 3) cardiac valve calcification; and 4) calciphylaxis or calcific uraemic arteriolopathy.24,50–52 Recently, it has been described a form of vascular calcification limited to the internal elastic lamina,53,54 and some authors have revelled the potential importance of myocardial calcification in inducing electrical disorders/sudden death.55,56
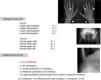
All arteries, even the smallest arterioles, may be affected by the calcification process; veins are rarely involved.50,57–60 For instance, the radial, cubital, and interdigital arteries are regulate the blood flow by changing the tone thanks to a dense layer of vascular smooth muscle cells which can be calcified.27,44,58,59 The wall of iliac and femoral arteries (predominantly but not exclusively made with vascular smooth muscle cells) are also more susceptible to develop calcification than the average.59 All these arteries are assessed by plain x-ray and they are used to calculate the Adragao score58 (Fig. 1). Another method for assessing calcification is the Kauppila score (Fig. 1) that evaluates the abdominal aorta, an elastic artery more susceptible for intimal calcification, given that elastic arteries (such as the subclavian and carotid arteries) have a media layer that contains more elastic fibres than muscle cells.27,44,50,54
Adragao Score: Plain x-ray of the hands and pelvis. Determined by the sum of the absence of calcification (0 points), unilateral (1 point) or bilateral (2 points) presence of linear calcifications in each section. It analyses calcification of the iliac, femoral, radial, and digital arteries. The final value ranges between 0 and 8 points (0–4 in the pelvis and 0–4 in the hands).58
Kauppila Score: A lateral abdominal x-ray is performed that includes from the T-10 vertebra to the first 2 sacral vertebra. The aorta is identified as a tubular structure in front of the vertebral column. Only the segments of the abdominal aorta that are in front of the first 4 lumbar vertebrae (L1-L4) are analysed. The score is assigned from 1 to 3 [1: small calcification (1/3 the vertebra length), 2: moderate (2/3), 3: large (affects more than 2/3 of the vertebrae length)] according to the length of each detected plaque. Both the anterior and posterior part of the aorta are taken into account, relating them with the location according to where they are located in front of the L1, L2, L3, or L4 vertebrae. With this scoring, a final score is reached between 0 and 24 points.135
Coronary artery calcification (CAC) has been described in 75% of the necropsies in the general population61 and in more than 95% of dialysis patients,62 other prevalence reports are variable (47–98%).63,64 The reported prevalence depends on the population, the vascular region being evaluated, and the sensitivity of the different diagnostic techniques used.63,65,66 Górriz et al. recently described that in CKD patients not on dialysis, besides the coronary calcifications,67 the vascular calcifications detected by hand and pelvic plain x-rays are a solid predictor of hospital-free survival, CV mortality, and overall mortality, even better that aortic calcification.59 This observation has important clinical implications that we will discuss in the second part of this review.29
Vascular and valve calcifications are more common and progress faster in chronic kidney disease: pathogenesis and implicationsCV calcification is not a new phenomenon nor it is exclusive to CKD.24,68 It is also common in diabetic patients and is closely correlated with age/ageing.13,27,48 Calcification is not a primary aetiological factor of arterial disease,19 since intimal calcification is part of the natural history of atherosclerosis at its late stages (stage vii), although it is associated with ischaemic CV events. Medial calcification, as an expression of arteriosclerosis, induces artery stiffness and increases the pulse wave velocity which contributes to development of left ventricle hypertrophy, fibrosis, ventricle dysfunction, decreased coronary irrigation during the diastole, and heart failure. The degree of calcification of each lesion has a variable relation with the severity of the associated stenosis; and, the relationship between the degree of calcification in an individual lesion and the probability of the plaque rupturing is unknown. Nevertheless, the presence of any type of vascular calcification is clearly associated with the degree of atheromatosis (which is in turn influenced by multiple factors such as age, dyslipidaemia, diabetes, CKD stage, time on dialysis, etc.), and with CV events, hospitalisation and mortality.27,59,69
The opinion of experts is not uniform,19,70–75 but it has not been confirmed that intimal calcification per se is a risk factor for plaque rupture and, it is well known that inflammation precedes the process of calcification and that they rarely overlap.76,77 However, this calcification seems to be directly associated with CV events.78,79 Even though calcification is a very late secondary phenomenon (progression of initial inflammation and atherosclerosis), there is not convincing evidence that calcification contributes to plaque stabilisation.80,81 Recent literature reveals that local tissue stress could increase due to the presence of juxtaluminal calcification and encrusted calcifications in the fibrous cap.81,82 Similarly, in CKD patients the atheroma plaque composition is characterised by an increase in calcification and a reduction in the amount of collagen which could lead to instability and plaque rupture.83,84 In any case, at least in haemodialysis patients, it must be taken into account that the most common cause of mortality does not seem to be plaque rupture, but rather non-atheromatous CV events such as sudden death (24.5% of total mortality in one study).55
The differential diagnosis of intimal vs medial vascular calcification, its relative importance, and the resulting clinical implications are widely debated.18,19,54,55,59,66 The different types of CV calcification lead to different clinical and prognostic features since they are associated to different types of CV events50; furthermore, its clinical expression is different in the general population vs CKD patients.54 Moreover, most CKD patients may present both types of vascular calcification simultaneously, with the potential overlap of pathological and clinical processes.27,28,50,59,62,85 It has been stated that both types of vascular calcification may be part of a continuum of a same vascular disease,22 and that medial calcification may have a higher prognostic value for identifying high-risk CKD patients.27
Vascular calcification is more prevalent and more severe in CKD patients, becoming more common as kidney function decreases.62,63,86 We recently described that vascular calcification, as assessed by plain x-ray, was already present in 79% of stage 3–5 CKD patients in Spain (67±13 years of age, 37% diabetic, creatinine 2.8±1.3mg/dl, mean glomerular filtration rate by MDRD 27±12ml/min/1.73m2, including 86% of stage 3–4 CKD patients).59 Vascular calcification was already prominent (defined as Adragao score≥3 or Kauppila score>6) (Fig. 1) in 47% of patients,59 and it was already known that it is more frequent in CKD patients in comparison to a control group.54 As previously mentioned, this accelerated progression of CV calcification is likely related to the atherosclerosis and rapid ageing affecting CKD patients which in turn could be attributed to multiple CKD-related factors: inflammation, changes to the nitric oxide pathway, oxidative stress, uraemic toxins, dialysis trauma, etc. or specific of CKD-MBD factors (P, Ca, etc.) that may act as powerful catalysers of CV calcification.18,19,21,87–91
There is extensive experimental evidence showing that several mineral metabolism disorders, part of CKD-MBD, promote CV calcification, especially the direct and indirect effect of P and Ca on vascular damage, independently of the passive precipitation of Ca and P in the vessel wall.89,92–95 A transformation from mesenchymal stem cells, pericytes, or vascular smooth muscle cells (VSMC) into cells similar to osteoblasts is also well documented, especially in uraemic conditions.28,92,96,97 Notably, a recent analysis of mammary arteries with vascular calcification in CKD patients found no evidence of osteogenic differentiation or apoptosis in the VSMC of these arteries, which indicates that the pathogenesis of medial calcification also differs among the different artery regions.98
Vascular calcification is also an active, highly organised, and well-regulated process that shares many similarities with bone formation and mineralisation. In 1575, Falloppio already described a transformation of arteries into bone, in what the physicians of the era called “ossification of the arteries”99; in 1863, Virchow described these vascular changes as “ossification, not mere calcification, occurring by the same mechanism by which an osteocyte builds calcium on the surface of bone”.100,101 Thus, it is not a surprise that there are factors in the vessel wall that impede a transformation into bone. In fact, not only local, but also systemic vascular calcification inhibitors have been described (including fetuin-A, matrix gla protein, pyrophosphates, etc.).102–104 In CKD patients, these inhibitors would be overwhelmed by a multitude of calcification promoters that favour calcification, doing so by inducing inflammation, oxidative stress, and even VSMC apoptosis. The final result is an unwanted imbalance in favour of procalcifying factors over calcification inhibitors that promote excessive vascular calcification.24,102 There are other factors that are directly or indirectly related to vessel wall damage and that promote CV calcification: proinflammatory cytokines, reactive oxygen species, bone morphogenetic proteins, uraemic toxins, micro-RNA, modulation of different cell signalling pathways (i.e. Wnt/β-catenin), or the novel role of calciproteins or elastolysis, which give rise to the complex physiopathology of CV calcification in CKD. A deep analysis of this highly complex process is far beyond the objectives of this manuscript; therefore, we refer the readers to other extensive reviews.19,24,28,96,97,105–109
Lastly, many studies have established several significant points that contribute to reinforce the importance of CV calcification in CKD patients: 1) vascular calcification represents an unquestionable marker of systemic vascular disease66; 2) there is a close correlation between CKD and CAC, even in young adults, thus this correlation is independent of age and atherosclerosis23; 3) there is a direct correlation between global and individual CAC and other sites of calcification with CV events, hospitalisation, and survival59,66,110–113; 4) baseline calcification is the most important individual prognostic factor for prediction of progression of CV calcification in kidney disease patients and some patients with no baseline calcification do not progress during follow-up24,114; 5) the progression of CAC has also provided additional prognostic information beyond the known risk factors and degree of initial calcification24,66; 6) CAC progression is associated with the worsening of markers of vascular and myocardial diseases66,115; and, lastly, 7) it has been recently demonstrated that the analysis of CAC improved the CV risk prediction model in CKD patients.116
Vascular calcification detection and clinical guidelinesAs mentioned, there is no doubt that the presence of any kind of CV calcification is associated with and adverse clinical outcomes. Nevertheless, there is debate in relation to the availability and the meaning of the different imaging techniques, quantification systems and scores, as well as whether it is possible to modify the progression of calcification. The 2009 published KDIGO clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of CKD-MBD did not recommend indiscriminate screening for vascular calcification of all CKD patients (a decision that was not unanimous).63 Subsequent recommendations by the U.S. National Kidney Foundation were similar to that of the KDIGO.117 However, it was also suggested that lateral abdominal x-rays could be used in stage 3-5D patients to detect the presence or absence of vascular calcification and that ultrasound could be used to detect the presence or absence of valve, calcification, all reasonable alternatives to the more expensive computed tomography-based methods (recommendation 3.3.1, 2C recommendation strength). Furthermore, the European Renal Best Practice (ERBP) working group considered that screening of incident dialysis patients was justified.118 The Spanish guidelines119 also consider baseline screening in all CKD patients justifiable with any technique (including vascular ultrasound), without being limited to the abdominal x-ray proposed by the international guidelines.54,120 In this sense, several observational studies, including the study by Górriz et al.,59 have confirmed that using plain x-rays in different areas is an effective, economical alternative for detecting vascular calcification and evaluating CV risk in the absence of a more specific CAC assessment.54,58,59,113,121,122 Moreover, we have emphasised the independent prognostic superiority of using the Adragao score ≥3 and even Adragao's hands only score >1 over the internationally recommended Kauppila lumbar score, even in non-dialysis CKD patients. Similarly, in this study, only the Adragao score, especially the hand score, maintained a correlation with the severity of kidney dysfunction, degree of secondary hyperparathyroidism, hospitalisation, and mortality,59 although, unlike others, vascular calcification was not necessarily associated with faster kidney function deterioration.59 Other authors had already indicated that in dialysis patients, digital artery calcification is a better predictor of mortality than abdominal aorta calcification.123 Lastly, it is important to note that the 2009 KDIGO guidelines proposed that stage 3-5D CKD patients with known vascular calcification be considered the group at the highest CV risk (guideline 3.3.2; 2A), and it was stated that “it is reasonable to use this information to guide the management of the CKD-MBD” (guideline 3.3.2; not graded).63
Even the above-mentioned ERBP guidelines stated that a vascular calcification assessment should be guaranteed in at least some patients, including “any patient in whom the caring physician decides that a knowledge of the presence of vascular calcification may impact therapeutic decision making”.118 Likewise the ERBP commentary on CKD-MBD considers, for example, that patients with vascular calcification “should receive little or no additional Ca-based phosphate binders” even though it recognises that there is a “continued and intense debate within the nephrological community” about the use of Ca-containing binders,118 as we will analyse in the second part of this review.29 In reality, vascular calcification is a component of CKD-MBD, and there are multiple experimental, epidemiological, and observational studies supporting that vascular calcification is not just a mere marker, but rather a direct cause of CV morbidity and mortality in CKD patients18; a post hoc analysis of recent studies in dialysis patients strengthens the plausibility of this hypothesis.55 However, some authors have the opinion that there is no clearly demonstrated treatment option that can be recommended once the presence of vascular calcification is detected.19,118 Results from a 2013 conference in Madrid on the controversies of the previous KDIGO guidelines has been published recently; the group unanimously stated that CV calcification should guide the treatment of CKD-MBD,124 but that there was no new sufficient evidence that would guarantee a reformulation of the statements made in the previous guidelines,63,124 even though several subsequent studies could strengthen them.65,125–129
Lastly, it is important to consider that while serum biomarkers reflect the risk to which an individual is exposed at the time of measurement,130 images of CV calcification represent the cumulative result of a prolonged exposure to one or multiple risk factors.66 In this way, different images and measurement methods have frequently shown that they are better predictors that the common J, inverted J, or U curve of the serological markers.59 For this reason, it is postulated that the images not only allows a better CV risk stratification,13,131 including patients with CKD,59,116,132 but also enable the use of personalised treatments, becoming a potential new clinical objective.66 Nevertheless, it becomes necessary to demonstrate beforehand that CV calcification is a modifiable risk factor with the possibility, at least, of decreasing its progression and not exacerbating it (if it cannot be reversed) as we will see in the second part of this review.29
ConclusionsCKD patients present a very high risk of CV disease and premature death; therefore we should offer them the opportunity to have the best prevention and treatment possible. In this context, we have demonstrated how the presence of CV calcification, a prominent characteristic of CKD-MBD, is directly associated with CV events and increased mortality in these patients. The evaluation of CV calcification is justified and it should be part of our protocols and future clinical studies since: it is a prominent characteristic of CKD-MBD, it is a superior predictor of clinical progression, it is a modifiable risk factor,29 and it can be used by nephrologists to make therapeutic decisions, even early in the evolution of their CKD.27,29,59,132–134
- •
CKD patients present a very high risk of cardiovascular disease and premature death, not always associated with atherosclerosis.
- •
Cardiovascular calcification is part of CKD-MBD.
- •
Vascular calcification and its progression are independent predictors of hospitalisation, cardiovascular events, and morbidity and mortality, in CKD patients even before starting dialysis.
- •
Vascular calcification progression is potentially modifiable.
- •
The 2009 KDIGO (and 2015 controversies) and 2011 Spanish guidelines consider using information on vascular calcification reasonable for guiding the management of CKD-MBD.
- •
Depending on the resources, vascular calcification assessment should be performed in any patient in whom the caring physician decides that information on the presence of vascular calcification may impact therapeutic decision.
- •
The Adragao score, in general, and the Adragao hand score, in particular, could emphasise the importance of controlling the CKD-MBD-associated factors in patients with vascular calcification.
No funding was received to complete this work.
Conflicts of interestDr Jordi Bover received conference honorariums from AbbVie, Amgen, Genzyme, and Shire, as well as consultation fees from AbbVie, Amgen, Vifor/Fresenius-Pharma, Chugai, Medice, and Genzyme/Sanofi. Dr José Luis Górriz received conference honorariums and grants from AbbVie. Dr Pablo Ureña received conference honorariums or consultation fees from Amgen, AbbVie, Genzyme-Sanofi, Medice, Hemotech, and Fresenius. Dr María Jesús Lloret received conference honorariums from Sanofi and AbbVie.
Dr Jordi Bover belongs to the Red Nacional RedinRen [National Kidney Research Network] (RD06/0016/0001 and RD12/0021/0033), the Red de Biobancos Nacional Española [Spanish National Biobank Network] (RD09/0076/00064), and to the Grupo Catalán de Investigación AGAUR [AGAUR Catalan Research Group] (2009 SGR-1116). Dr Jordi Bover collaborates with the Fundación Iñigo Álvarez de Toledo (FRIAT) [Iñigo Álvarez Foundation of Toledo]. We also wish to thank Ricardo Pellejero for his important bibliographic help.
Please cite this article as: Bover J, Górriz JL, Ureña-Torres P, Lloret MJ, Ruiz-García C, daSilva I, et al. Detección de las calcificaciones cardiovasculares: ¿una herramienta útil para el nefrólogo?. Nefrología. 2016;36:587–596.




![Adragao Score: Plain x-ray of the hands and pelvis. Determined by the sum of the absence of calcification (0 points), unilateral (1 point) or bilateral (2 points) presence of linear calcifications in each section. It analyses calcification of the iliac, femoral, radial, and digital arteries. The final value ranges between 0 and 8 points (0–4 in the pelvis and 0–4 in the hands).58 Kauppila Score: A lateral abdominal x-ray is performed that includes from the T-10 vertebra to the first 2 sacral vertebra. The aorta is identified as a tubular structure in front of the vertebral column. Only the segments of the abdominal aorta that are in front of the first 4 lumbar vertebrae (L1-L4) are analysed. The score is assigned from 1 to 3 [1: small calcification (1/3 the vertebra length), 2: moderate (2/3), 3: large (affects more than 2/3 of the vertebrae length)] according to the length of each detected plaque. Both the anterior and posterior part of the aorta are taken into account, relating them with the location according to where they are located in front of the L1, L2, L3, or L4 vertebrae. With this scoring, a final score is reached between 0 and 24 points.135](https://static.elsevier.es/multimedia/20132514/0000003600000006/v1_201702210030/S2013251416301481/v1_201702210030/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)




