Sarcopenia and dynapenia are two terms associated with ageing that respectively define the loss of muscle mass and strength. In 2018, the European Working Group on Sarcopenia in Older People (EWGSOP) introduced the EWGSOP2 diagnostic algorithm for sarcopenia, which integrates both concepts. It consists of 4 sequential steps: screening for sarcopenia, examination of muscle strength, assessment of muscle mass and physical performance; depending on these last 3 aspects sarcopenia is categorised as probable, confirmed, and severe respectively. In the absence of validation of the EWGSOP2 algorithm in various clinical contexts, its use in haemodialysis poses several limitations: (a) low sensitivity of the screening, (b) the techniques that assess muscle mass are not very accessible, reliable, or safe in routine clinical care, (c) the sequential use of the magnitudes that assess dynapenia and muscle mass do not seem to adequately reflect the muscular pathology of the elderly person on dialysis. We reflect on the definition of sarcopenia and the use of more precise terms such as “myopenia” (replacing the classic concept of sarcopenia to designate loss of muscle mass), dynapenia and kratopenia. Prospective evaluation of EWGSOP2 and its comparison with alternatives (i.e. assessment of kratopenia and dynapenia only; steps 2 and 4) is proposed in terms of its applicability in clinical routine, resource consumption, identification of at-risk individuals and impact on events.
Sarcopenia y dinapenia son dos términos asociados al envejecimiento que definen respectivamente la pérdida de masa y de fuerza muscular. En el año 2019, el European Working Groupon Sarcopenia in Older People (EWGSOP) introdujo el algoritmo diagnóstico EWGSOP2 de sarcopenia, que integra ambos conceptos. Consiste en 4 pasos secuenciales: cribado de sarcopenia, exploración de la fuerza muscular, evaluación de la masa muscular y de su rendimiento físico; dependiendo de estos 3 últimos aspectos la sarcopenia se categoriza como probable, confirmada y grave respectivamente. A falta de validación del algoritmo EWGSOP2 en diversos contextos clínicos, su utilización en hemodiálisis plantea diversas limitaciones: (a) poca sensibilidad del cribado, (b) las técnicas que evalúan la masa muscular son poco accesibles, fiables o seguras en la rutina clínica asistencial, (c) el uso secuencial de las magnitudes que evalúan la dinapenia y la masa muscular no parecen reflejar adecuadamente la patología muscular del anciano en diálisis. Reflexionamos sobre la definición de sarcopenia y la utilización de términos más precisos como “miopenia” (sustituyendo al concepto clásico de sarcopenia para designar la pérdida de masa muscular), dinapenia y kratopenia. Se propone la evaluación prospectiva del EWGSOP2 y su comparación con alternativas (ej. evaluación exclusiva de kratopenia y dinapenia; pasos 2 y 4) en cuanto a su aplicabilidad en la rutina clínica, consumo de recursos, identificación de personas en riesgo e impacto sobre eventos.
Although the term sarcopenia was introduced quite a few years ago, it is currently in vogue, due to its clinical implication with frailty and dependency, especially in the geriatric or inflamed patient. However, it remains a confusing and unclear term in which overlapping concepts converge.
Definition and evolution of the concept of sarcopeniaThe word sarcopenia is derived from Greek and means scarcity (penia) of flesh (sarx). Rosenberg first used the term sarcopenia in 1988, to identify a clinical condition characterized by the loss of skeletal muscle mass in the context of aging.1 The concept of sarcopenia refers exclusively to skeletal muscle, not including the other three types of muscle tissue: smooth muscle, myocardium and myoepithelium of certain glands. It is important to remember that each skeletal muscle fiber is a syncytium where many nuclei share the same cytoplasm, as a result of the fusion of several myoblasts. Therefore, it loses the ability to divide and, once formed, the skeletal muscle fiber cannot reproduce, although it can increase (hypertrophy) or decrease (atrophy) in size or volume.
DynapeniaSubsequently, there was the need to evaluate the decrease in strength, in addition to the loss of muscle mass, as well as its relationship with the loss of physical function, mortality, quality of life and disability.2–4 Clark et al. introduced the term dynapenia, as loss of muscle mass, arguing that it is a concept independent of sarcopenia and whose pathogenesis may not be due solely to the decrease in muscle mass.5,6 Since then, various groups and associations have tried to reach a consensus definition of sarcopenia7–11; all of them always include muscle mass, some include strength and most include physical performance.
MyopeniaMyopenia is the loss of muscle mass. It is measured by bioimpedance (BIA), dual energy X-ray absorptiometry (DEXA) or magnetic resonance imaging (MRI) and computed tomography (CT).
KratopeniaKratopenia or muscle power deficit is another important concept related to sarcopenia, which should be integrated into the definition in order to better understand the defect attributed to the muscle. Kratopenia has been defined as the loss of muscle contraction capacity measured by dynamometry or isotonic contraction test (Table 1).
Definitions of the concepts included in skeletal muscle disorders.
| Sarcopenia = skeletal muscle disease that causes loss of muscle mass and strength. |
| Kratopenia = deficit of muscle power; It is measured with dynamometry or isotonic contraction test. |
| Myopenia = Loss of muscle mass. It is measured by bioimpedance (BIA), dual energy X-ray absorptiometry (DEXA) or magnetic resonance imaging (MRI) and computed tomography (CT). |
| Dynapenia = Loss of muscle strength. Measured by walking speed test, distance or rising from a chair. |
Sarcopenia is a syndrome fundamentally associated with old age,12 and in fact, in 2016 it was included as a disease in the international classification of diseases (ICD-10, in its MC version [Clinical Modification]) with the code M62.84,13,14 while dynapenia is not incorporated.
Evolution of the diagnostic algorithm EWGSOP1 to EWGSOP2The most active and recognized group in the study of sarcopenia is the European Working Group on Sarcopenia in Older People (EWGSOP), which in 201012 proposed the following criteria for diagnosing sarcopenia:
- -
Criterion 1: Low muscle mass (mandatory criterion).
- -
Criterion 2: Reduced muscle strength.
- -
Criterion 3: Low physical performance.
These criteria allow to establish a classification of sarcopenia according to its degree of severity as:
- -
Mild sarcopenia or pre-sarcopenia: presence of criterion 1 (low muscle mass).
- -
Moderate sarcopenia: presence of criterion 1 in addition to 2 or 3 (low muscle mass + reduced muscle strength or decreased physical performance).
- -
Severe sarcopenia: presence of all three criteria (low muscle mass + reduced muscle strength or decreased physical performance).
In January 2019, the EWGSOP revised and updated its definition of sarcopenia.15 New features provided by the new consensus (EWGSOP2) are:
- A)
Sarcopenia is no longer considered a geriatric syndrome, but rather a skeletal muscle disease (muscle failure) that is not always associated with aging, as it can appear in younger people16 and may be due to causes other than aging itself.17,18
- B)
The concept of muscle quality is introduced, so that the decrease in strength becomes important in the diagnosis.19–21 This approach leads to the presentation of grip strength as the first element in the diagnosis of sarcopenia. The patient with low muscle strength is classified as probably sarcopenic, and the diagnosis is subsequently confirmed by measuring muscle mass.
- C)
Impairment of physical performance or functional capacity is now considered a criterion of disease severity. It can be measured by tests such as gait speed (MV), the ability to get up from a chair, walk and sit down again or the battery of tests in which a balance assessment is included.4,22–24
- D)
Sarcopenia is recognized as an underdiagnosed and therefore undertreated condition,25 possibly because of the varied diagnostic criteria and different cut-off points. Seeking a clinical utility of the diagnostic algorithm, new well-defined cut-off points are presented and their clinical study in special populations is proposed.
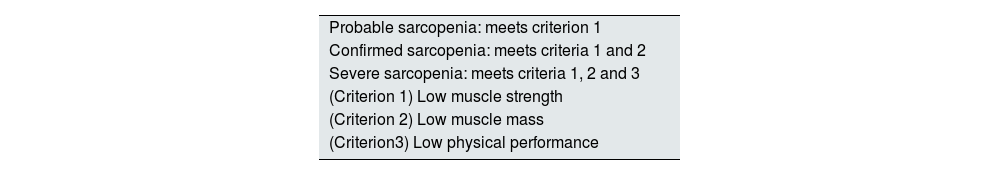
According to the new EWGSOP consensus2 (Table 2), when low muscle strength is detected (criterion 1), sarcopenia is probable; the diagnosis is confirmed if low muscle mass is found (criterion 2) and it is considered severe or serious sarcopenia if, in addition, physical function or performance is diminished (criterion 3). In any case, no different terms are specified to define loss of strength or loss of muscle mass, and all are referred to as sarcopenia with nuances (probable, confirmed or severe).
Definition of sarcopenia according to EWGSOP2.
| Probable sarcopenia: meets criterion 1 |
| Confirmed sarcopenia: meets criteria 1 and 2 |
| Severe sarcopenia: meets criteria 1, 2 and 3 |
| (Criterion 1) Low muscle strength |
| (Criterion 2) Low muscle mass |
| (Criterion3) Low physical performance |
Modified from Cruz-Jentoft et al.15
EWGSOP2: new consensus of the European Working Group on Sarcopenia in Older People.
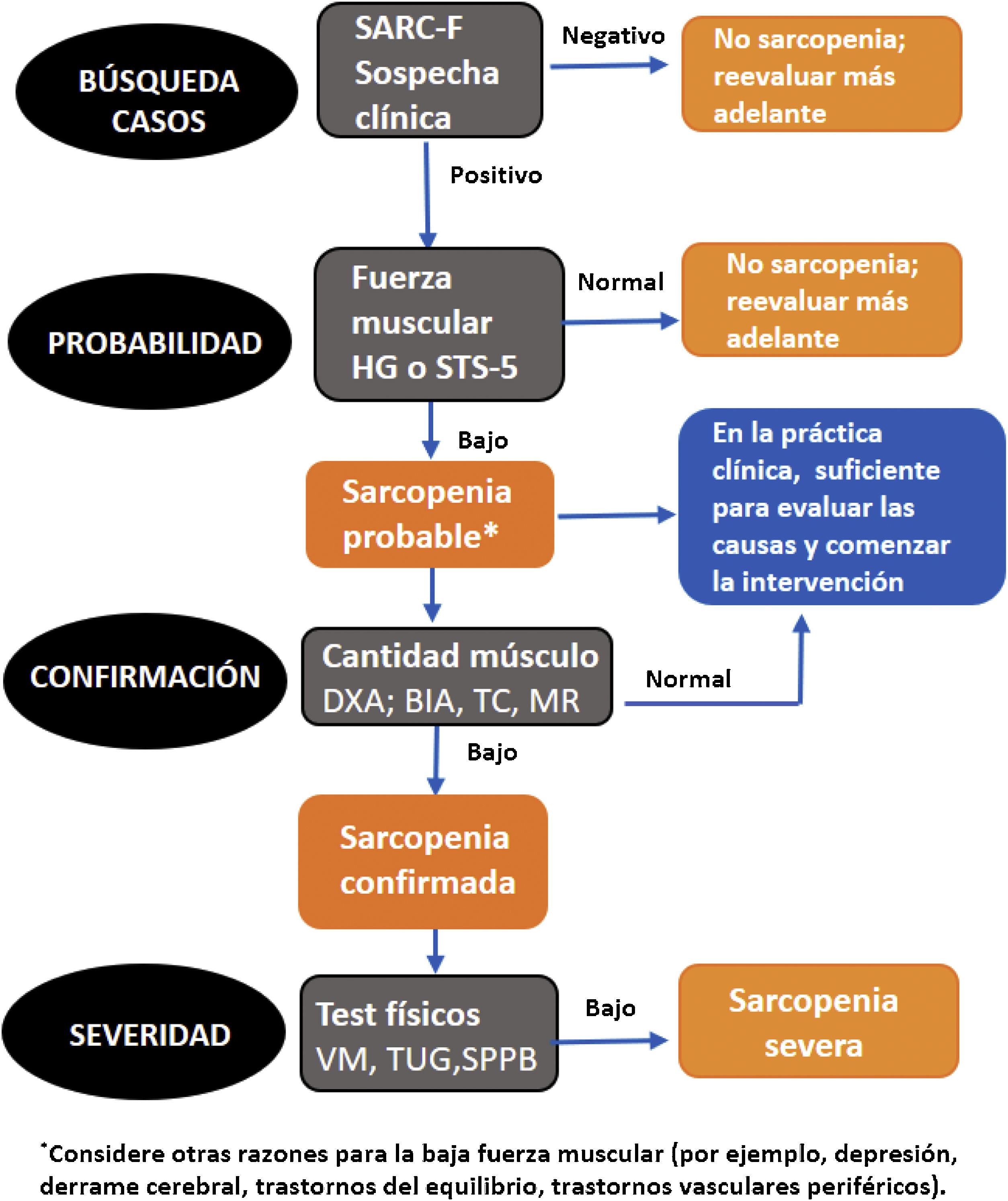
The new EWGSOP2 algorithm for the detection and diagnosis of sarcopenia includes four phases or stages (Fig. 1).15
- 1
Case finding: Survey on Strength, Assistance walking, Rise from a chair, Climb stairs, and Falls (SARC-F) used in all classifications as a screening test for sarcopenia.26
- 2
Probability assessment: determination of strength.
- 3
Confirmation: measurement of muscle mass.
- 4
Assessments of severity: physical test performance27,28 (Fig. 1).
- 5
Screening is recommended to establish clinical suspicion, using the SARC-F questionnaire that evaluates the appearance of symptoms such as weakness, slowness, falls or difficulty in performing usual daily activities.26 This is a very easy-to-apply survey that assesses the difficulty or not that a subject presents in carrying a weight, walking across a room, getting up from a bed or chair and climbing a flight of 10 steps, as well as the number of falls he/she has suffered in the last year. The total score can range from 0 to 10 (none to maximum difficulty) and each domain scores from zero to two points. A total score ≥4 predicts sarcopenia. This survey has a low sensitivity, but excellent specificity.29,30 However, it is surprising that a low sensitivity tool is used for screening: screening tests usually emphasize sensitivity, because subsequent confirmatory tests will provide specificity.
- 6
The determination of muscle strength can be performed in the upper body by using manual dynamometry (HG) or in the lower body by performing the sit to stand to sit 5 (STS-5) test.
- -
HG test: this test assesses manual grip strength using a dynamometer. To perform it, the patient must be in a standing position, with the arm slightly apart and along the body. The test is performed twice with each arm, and the maximum score obtained is considered.
- -
STS-5: the purpose of these tests is to assess the strength of the lower extremities. The STS-5 consists of counting the time it takes the patient to perform five repetitions of standing up and sitting on a chair.
- -
- 7
Several methods have been proposed to measure muscle mass:
- -
DEXA: this is a widely used and recommended method.31 It has minimal radiation exposure, but since it is not portable, its use is limited in non-hospital centers.
- -
MRI and CT: considered the gold standard in non-invasive muscle mass measurement. However, its high cost and lack of portability reduce its use. In addition, it requires highly trained personnel to use the equipment, the cut-off points that establish low muscle mass are not yet well defined,32 access to MRI is limited in some settings, and CT exposes patients to radiation.
- -
BIA (Bioimpedancia): unlike the previous method, this is a cheap, easy and portable technique that can be performed at the bedside or in ambulatory patients. Its disadvantage is that it does not measure muscle mass directly, but estimates it from the electrical conductivity of the whole body, and measurements can be influenced by the subject's state of hydration and other circumstances.28,33
- -
- 8
When muscle mass is low, EWGSOP2 recommends functional tests to assess its severity, including the measurement of MV, The Timed-Up and Go test (TUG) or the Short Physical Performance Battery (SPPB).27
- -
MV: measured as the time required to walk four meters and expressed in meters per second, taking into account whether assistance was required to maintain balance during the walk (cane, walker, other hand).
- -
SPPB or Guralnik test: consists of five physical tests, three balance tests, one MV test and one lower body strength test.
- -
TUG: this test assesses agility and dynamic balance. In this test, the patient must get up from a chair, walk a distance of three meters, turn around a cone and sit down again. This is done at the maximum walking speed. The test is performed in three attempts, with the shortest time remaining as the final result.
- -
Modified from Cruz-Jentoft et al.15 AMS: appendicular muscle mass; HG: dynamometric grip strength; SARC-F: Sarcopenia Screening Survey; SPPB: Short Physical Performance Battery; STS-5: sit to stand to sit 5 test; TUG: The Timed-Up and Go test; VM: walking speed.
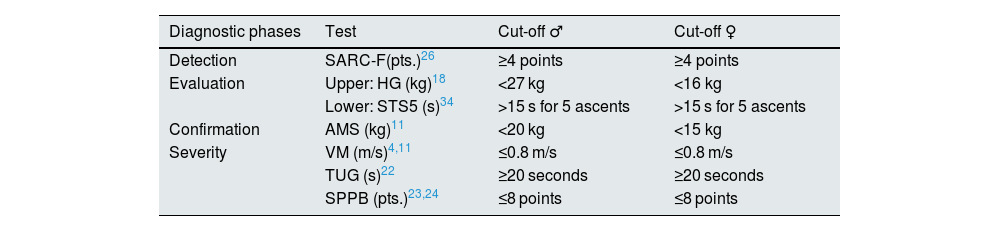
The cut-off points for the different parameters defined by the EWGSOP2 are shown in Table 3.
Cut-off points marked for the diagnosis of sarcopenia.
| Diagnostic phases | Test | Cut-off ♂ | Cut-off ♀ |
|---|---|---|---|
| Detection | SARC-F(pts.)26 | ≥4 points | ≥4 points |
| Evaluation | Upper: HG (kg)18 | <27 kg | <16 kg |
| Lower: STS5 (s)34 | >15 s for 5 ascents | >15 s for 5 ascents | |
| Confirmation | AMS (kg)11 | <20 kg | <15 kg |
| Severity | VM (m/s)4,11 | ≤0.8 m/s | ≤0.8 m/s |
| TUG (s)22 | ≥20 seconds | ≥20 seconds | |
| SPPB (pts.)23,24 | ≤8 points | ≤8 points |
AMS: muscle mass; HG: grip strength with dynamometry; TUG: The Timed-Up and Go test; SARC-F: sarcopenia screening survey; SPPB: Short Physical Performance Batter; STS-5: sit to stand to sit 5 test; VM: walking speed.
In clinical practice EWGSOP2 proposes to classify sarcopenia according to its etiology, distinguishing between:
- -
Primary sarcopenia, age-related, when no cause other than aging itself is determined.
- -
Secondary sarcopenia, when causes other than age are observed. It can appear secondarily in the context of various systemic diseases, in cardiac or respiratory failure, in renal disease, and especially in those pathologies involving inflammatory processes. Physical inactivity is a factor that also contributes to the development of sarcopenia, either due to a sedentary lifestyle or due to immobility or disability related to the disease.35,36 Similarly, inadequate energy or protein intake, as a result of anorexia, malabsorption, limited access to healthy foods, or a limited ability to eat, can be a cause of sarcopenia.
Chronic kidney disease (CKD) is a growing problem in the world’s healthcare system. It is defined as the loss, for at least three months, of renal function, established by a reduction in glomerular filtration rate of less than 60 mL/min/1.73 m2 or by the presence of renal damage verified directly by renal biopsy or indirectly by the presence of albuminuria, alteration in the urine sediment or by imaging techniques.37
In Spain, the incidence of CKD is increasing mainly due to the aging of the population and the increase in pathologies considered risk factors such as diabetes mellitus, cardiovascular disease or obesity.38,39 According to the Spanish Registry of Renal Patients (REER),40 the incidence of kidney patients starting renal replacement therapy has increased from 121.1 to 141.4 per million population (pmp) in the last 10 years. Of the 5,817 deaths in 2020, recorded in the REER, 44% corresponded to those over 75 years of age. CKD is associated with an increased risk of cardiovascular and/or all-cause mortality at all ages,41 with sarcopenia being an indicator of mortality.42
The prevalence of sarcopenia in CKD varies depending on the diagnostic criteria used and the characteristics of the patients studied. This variability has also been seen in hemodialysis (HD) subjects, ranging from 4 to 64%.43–45 Even so, it is a frequent pathology in renal patients and its prevalence increases notably as renal function decreases.46 We observed a prevalence according to EWGSOP2 of 20% in elderly HD patients, which is 75-95% when muscle performance is assessed without taking mass into account.30
The causes of sarcopenia in patients with CKD are diverse and its consequence is the imbalance between muscle synthesis and catabolism. The term uremic sarcopenia has even been coined to define this situation in renal patients.47–49 The increasing age of subjects with renal disease favors the presence of associated comorbidities that lead to inactivity28,30 and hospitalization.50 Other risk factors also contribute, such as malnutrition, due to decreased intake due to severe dietary restrictions or the use of medications that reduce appetite, and increased nutrient losses during dialysis itself.51 Metabolic acidosis, accumulation of uremic toxins and proinflammatory cytokines, and the dialysis procedure itself accelerate protein catabolism and loss of lean mass.52 Insulin resistance, hormonal imbalance, vitamin D deficiency and oxidative stress in the renal patient potentiate the incidence of sarcopenia.53–55 Simultaneously, hypogonadism56 and the accumulation of uremic toxins that alter muscle mitochondrial function57 affect muscle regeneration, favoring the loss of muscle strength in CKD.
Along with the decrease in the amount of muscle, impaired renal function is associated with selective changes in muscle structure with a significant reduction in muscle strength.58,59 This is important as there is not always a linear relationship between muscle mass and muscle strength. Muscle strength, power and performance result from the integration of components, including size, fiber type, quality and innervation. Therefore, even in the absence of sarcopenia defined as loss of muscle mass, it is possible to have dynapenia, established as muscle weakness that limits activities of daily living. The diagnosis of dynapenia would focus on identifying a loss of muscle strength or power, regardless of muscle size.60 Our studies show that exercise improves both muscle strength and muscle mass, although it is more relevant in lower limb strength as corresponds to the type of exercise performed.28
Our group has observed that in geriatric patients on HD, there may be normal muscle mass, with a loss of strength,30 meeting only in part the EWGSOP2 criteria for sarcopenia.
The EWGSOP2 definition of sarcopenia includes the concepts of myopenia and dynapeniaThe EWGSOP2 definition of sarcopenia is the disease of skeletal muscle with loss of muscle mass and strength. This concept integrates under the term sarcopenia the initial concept of sarcopenia (loss of muscle mass) and dynapenia (reduction of strength) without clarifying whether a muscle of normal mass, but with low strength, can be accepted as sarcopenia. In other words, muscle disease and loss of muscle mass are identified under the same name of sarcopenia, although they are not the same concept. To solve the problem, it has been proposed to call myopenia the deficit of muscle mass.61 It is important to use a precise language that allows information to be exchanged and to identify whether it is a loss of strength, muscle mass or both. Therefore, sarcopenia would be equal to myopenia (muscle mass deficit) + dynapenia (decreased muscle strength). In our experience, the renal patient may have an acceptable muscle mass, with a loss of strength28,30,62 so the EWGSOP2 definition of sarcopenia does not adequately capture his muscle pathology.
KratopeniaKratopenia or muscle power deficit is another important concept related to sarcopenia, which should be integrated into the definition in order to better understand the defect attributed to the muscle. As mentioned above, kratopenia has been defined as the loss of muscle contraction capacity measured by dynamometry or isotonic contraction test (included as a probability of sarcopenia according to EWGSOP2), while dynapenia would be the loss of strength measured by MV, SPPB or chair stand-up test (included in the EWGSOP2 concept of severity)15 (Table 2). The integration of the term kratopenia would allow sarcopenia to be defined as the simultaneous presence of myopenia (muscle mass deficit) + dynapenia (decreased muscle strength) + kratopenia (reduced power) (Table 4). However, according to EWGSOP2, sarcopenia would be myopenia + kratopenia exclusively, and would be classified as severe when dynapenia is present.
The EWGSOP2 definition of sarcopenia includes the concepts of kratopenia, myopenia and dynapenia, although it is not explicitly stated as such.
| Severe sarcopenia = kratopenia (loss of muscle power) + myopenia (loss of muscle mass) + dynapenia (loss of muscle strength) |
EWGSOP2: new consensus of the European Working Group on Sarcopenia in Older People.
Sarcopenia according to EWGSOP2 is the disease of skeletal muscle that includes muscle mass and strength. However, there may be only loss of muscle mass or strength and there are terms that allow defining different components included in the EWGSOP2 concept of sarcopenia: kratopenia is the loss of power, myopenia is the deficit of muscle mass and dynapenia is the decrease of strength. Therefore, sarcopenia would be the sum of kratopenia and myopenia, and severe sarcopenia would also add dynapenia. We propose a reflexion on the definition of sarcopenia. Consideration should be given to increasing precision in the language by incorporating the concepts of myopenia (loss of muscle mass, it would replace the original concept of sarcopenia), kratopenia and dynapenia. In addition, EWGSOP2 should be prospectively assessed and compared with alternatives (e.g., assessment of kratopenia and dynapenia only, and using steps 2 and 4) in terms of its applicability in clinical routine, resource consumption, identification of at-risk individuals, and impact on outcomes. The renal patient may have normal muscle mass, with a loss of strength and even power caused by multiple factors. Therefore, if the results of these prospective studies differ for CKD subjects on HD from other populations, consideration should be given to replacing the term sarcopenia with another term that reflects the peculiarity of CKD muscle disease, such as “uremic myopathy”.
FinancingThe authors have had no funding of any kind in the present writing. The authors declare that they currently hold the following state grants. The research groups of E.G.P., S.M.F. and A.O. are funded by the Ministry of Economy, Industry and Competitiveness: FIS/FEDER funds (PI21/01430, PI16/01298, PI18/01386, PI19/00588, PI19/00815, PI20/00487, PI21/01240 and DTS18/00032), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064, PERSTIGAN AC18/00071 and ISCIII-RETIC REDinREN RD016/0009) and Sociedad Española de Nefrología, Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM.
Conflicts of interestA. Ortíz has received grants from Sanofi and consulting or speaker’s fees or travel support from Advicciene, Astellas, AstraZeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk and Vifor Fresenius Medical Care Renal Pharma and is director of the Mundipharma-UAM Chair in diabetic kidney disease and the AstraZeneca-UAM Chair in chronic kidney disease and electrolytes. A. Ortíz is the editor-in-chief of CKJ (until May 21, 2022). The rest of the authors have no conflicts of interest.