Contrast-induced encephalopathy is a neurological complication related to contrast used in endovascular procedures or computed tomography (CT). The main risk factors are arterial hypertension, diabetes mellitus, chronic kidney disease (CKD), hyperosmolar contrasts, the amount of infused contrast and its direct infusion in the posterior cerebral territory, or pathologies with blood-brain barrier damage. Symptomatology is non-specific and may present as altered level of consciousness, neurological focality or seizures. Diagnosis is done by exclusion after ischemic or hemorrhagic stroke has been ruled out; CT or MRI are useful for differentiation. Generally, it appears shortly after exposure and the symptoms lasts 48−72h with complete recovery, although cases with persistence of symptoms or longer duration have been described. Treatment consists of monitoring, supportive measures and kidney replacement therapy (KRT) with hemodialysis (HD) in patients in chronic KRT program. It is important for the nephrologist to be aware of this entity given the susceptibility of the patient on HD as well as its potential therapeutic role in these patients.
La encefalopatía por contraste es una complicación neurológica relacionada con el contraste utilizado en procedimientos endovasculares o tomografía computarizada (TC). Los principales factores de riesgo son la hipertensión arterial, la diabetes mellitus, la enfermedad renal crónica (ERC), contrastes hiperosmolares, cantidad de contraste infundida y su infusión directa en el territorio cerebral posterior, o patologías que cursen con daño de barrera hematoencefálica. La sintomatología es inespecífica y puede presentarse como alteración del nivel de conciencia, focalidad neurológica o crisis comiciales. El diagnóstico es de exclusión tras haber descartado los accidentes cerebro-vasculares isquémicos o hemorrágicos, el TC o la resonancia magnética son de utilidad para su diferenciación. Generalmente, aparece poco tiempo tras la exposición y la sintomatología dura 48−72h con recuperación completa, aunque se han descrito casos con persistencia de los síntomas o mayor duración. El tratamiento es la monitorización con medidas de soporte y la terapia de sustitución renal con hemodiálisis (HD) en aquellos pacientes en programa crónico. Es importante que el nefrólogo conozca esta entidad dada la susceptibilidad del paciente en HD así como su potencial papel terapéutico en estos pacientes.
Contrast-induced encephalopathy (CIE), also called contrast-induced neurotoxicity, is a central nervous system (CNS) pathology secondary to toxicity resulting from exposure to radiological contrasts in patients undergoing diagnostic or therapeutic procedures that require its use (e.g., cerebral angiography, computed tomography [CT], cardiac catheterization).
Patients with advanced chronic kidney disease (ACKD), stages 4–5 according to the Kidney Disease Improving Global Outcomes (KDIGO) classification,1 with estimated glomerular filtration rate (eGFR) below 30 mL/min, are at a risk for the related toxicity of these agents, such as contrast induced nephropathy, due to their lower renal clearance.2 Cases of CIE have been reported in renal replacement therapy in hemodialysis2,3 (HD) patients, as well as in CKD patients without specifying the eGFR.4
The first studies dealing with this type of neurotoxicity were published in the 1960s in patients undergoing cerebral angiography,5 which due to its clinical presentation were named "transient brain blindness" (TBB). It was later described by specialists outside neurology, but with common use of radiological contrast, specifically after cardiac catheterization4 or CT.6
The clinical presentation is varied, ranging from altered level of consciousness to neurological focality or seizures (Table 1). Onset is early (minutes to hours) after exposure to the contrast medium and generally resolves within 48−72 h. Due to the higher natural permeability of the blood-brain barrier (BBB) in the posterior cortex,7 visual symptoms are the most frequently reported in the literature. Some authors postulate that in ACKD patients the clinical presentation is different,8 but there are no comparative studies showing such a difference.
Clinical manifestations of contrast-induced encephalopathy. Adapted from Viganò et al.11.
| Symptoms | Frequency (%) |
|---|---|
| Encephalopathy | 39.6 |
| Cortical blindness | 39.6 |
| Unilateral motor deficits | 37.5 |
| Drop of attention | 20.8 |
| Aphasia | 18.8 |
| Headache | 18.8 |
| Seizures | 16.7 |
| Neglect syndrome | 12.5 |
| Unilateral hemianopsia | 12.5 |
| Dysautonomia | 10.4 |
The presentation is infrequent and the exact incidence is unknown. A classic study from the 1970s estimates an incidence of 3.6% of all cerebral angiographies9; however a more recent review by the group of Li et al. that evaluates cases with CIE in all patients undergoing cerebral angiography during a 10-year period in five different centers in China estimates an incidence of 0.3–1%.10 It has been described after angiographic procedures in different vascular territories (supra-aortic, spinal, thoracic, coronary and abdominal trunks), but cerebral procedures have the highest risk.11
Pathophysiology and risk factorsThe most widely accepted hypothesis is direct neuronal toxicity following BBB disruption. The exact mechanisms are unknown, but it is postulated that the hyperosmolarity of these agents produces vasodilatation, contraction of the cerebrovascular endothelium and opening of the endothelial cell junctions. This was shown by Rapoport’s experiments using hyperosmolar solutions with mannitol infused into the carotid territory of mices12 facilitating the direct toxic effect of the contrast to neurons which, together with the hyperosmolar environment, leads to brain edema. Currently most of the contrasts used are hypoosmolar (iopamidol or iohexol, among others) or isoosmolar (mainly iodixanol), with a hypothetical advantage over hyperosmolar contrasts, despite the fact that the so-called "hypoosmolar" contrasts have a higher osmolality than blood. However, there are no comparative studies between hyperosmolars and the rest, nor between hypoosmolars and isoosmolars. In the case of hypoosmolar contrasts, the difference in osmolalities would affect the BBB and in the case of isoosmolar contrasts, in the absence of this difference in osmolality, a greater toxicity is assumed in cases of BBB damaged due to the presence of other risk factors, together with a possible direct toxic effect on the endothelium.
Regarding the risk factors related to this entity, kidney insufficiency has been described as a risk factor due to its theoretical lower clearance of the agents used. In this regard, a work by the Australian group of Spina et al.4 reviewed all cases of CIE after cardiac catheterization up to 2017, and it was found that CKD was present in 15% (8/52) of cases, behind arterial hypertension (HTN) in 57.7% (30/52) and diabetes mellitus in 25% (13/52). Another study by the Japanese group of Matsubara et al. that reviewed all cases of CIE after embolization of intracranial aneurysms in their center over a period of 10 years, found an incidence of 38% (3/8) in patients on HD, postulating an added risk of increased exposure due to the absence of kidney elimination.3 The main limitation was that patients with CKD who were not on chronic HD were not included because the procedure was not performed due to the risk of worsening renal function. Finally, the study by Chu et al.13 does find statistically significant results regarding the role of CKD as a risk factor for this pathology. They found a difference of up to 20 mL/min between groups when analyzing the incidence of CIE in patients with acute ischemic stroke who underwent endovascular thrombectomy over 5 years in two tertiary centers in Taiwan; however, they do not categorize CKD to see whether advanced stages pose a greater risk, nor do they establish a threshold value.
Regarding other risk factors, the study by Li et al.10 found no significant effects of HTN or DM as risk factors for CIE in patients undergoing cerebral angiography. The same authors assume the possible relevance of chronic HTN due to its negative effect on the BBB function, demonstrated in other pathologies such as posterior reversible encephalopathy syndrome (PRES), affecting cerebral arterioles and promoting ischemia and vasogenic edema.14 In their work, Li et al. found a prevalence of 55.6% of HTN in the CIE group, as opposed to 36.8% in the control group; however the difference did not reach statistical significance. With respect to DM they found a prevalence of 11.1% in CIE patients as compared to 6.8% in the control group, again, without statistical significance but supporting the theory of a greater susceptibility to ischemia and cerebral edema secondary to episodes of recurrent hypoglycemia, with the increase in intracellular electrolytes during these episodes, as in diabetic ketoacidosis.15 In this regard, a 1978 study from America using positron emission tomography with18 F-fluorodeoxyglucose showed that the posterior cortex has higher metabolic rates than other areas of the brain,16 which would explain the greater susceptibility of this area of the CNS to the compromise of glucose supply due to harmful stimuli.
The study by Li et al.10 has 2 limitaations that should be noted. First, only 18 patients (0.35%) presented CIE, and patients who presented encephalopathy without blindness were not included; second, patients with renal insufficiency were excluded and the role of CKD as a risk factor was not analyzed. The only statistically significant factors they found were the dose of contrast administered and its direct administration into the cerebral posterior circulation.
Other patient-related factors described are autoimmune diseases due to possibility of vasospasm and vasogenic edema in the cerebral territory, that would increase the susceptibility to contrast neurotoxicity,10 however there are no specific studies in this regard. CIE appears to be an idiosyncratic reaction, thus repeated exposure should not be a risk factor for developing CIE.17
Regarding factors related to the technique or the contrast used, only Li et al.10 found a significant effect regarding the direct infusion over the posterior territory as compared to the anterior cerebral circulation; although prior to the study by Li there was a belief of increased risk due to the greater permeability of the BBB in this territory. This Group (Li et al.10) finds an association between CIE with the use of higher doses of contrast, but there are no studies that recommend a maximum amount, although they propose a dose of no more than 200 cc or 300 cc in case of systemic administration,18 emphasizing that intracranial infusion should be performed using lower doses.19 However, there are reported cases of CIE with up to 25 cc infused in carotid territory.20 Finally, radiological contrasts are classified according to their ionic composition and osmolality. There are no studies comparing the incidence of CIE with ionic and non-ionic contrasts (more hypoosmolar, Table 2). Theoretically, hypoosmolar and non-ionic contrasts have a lower risk of developing CIE, but most of the cases described correspond to this type of agents, as they are the most commonly used in these procedures.20
Characteristics of some contrast agents. Comparison of osmolality with respect to blood osmolality. Adapted from Spina et al.4
| Contrast agent | Class | Iodine concentration (mg I/mL) | Osmolality (mOsm/kg water) |
|---|---|---|---|
| Diatrizoate | Monomer-ionic | 370 | 2.100 |
| Iotalamate | Monomer-ionic | 400 | 2.400 |
| Iohexol | Non-ionic monomer | 350 | 915 |
| Iomeprol | Non-ionic monomer | 350 | 610 |
| Iopamidol | Non-ionic monomer | 370 | 774 |
| Iopromide | Non-ionic monomer | 350 | 774 |
| Ioversol | Non-ionic monomer | 320 | 790 |
| Ioxaglate | Dimer-ionic | 320 | 577 |
| Iodixanol | Non-ionic dimer | 320 | 290 |
| Blood | – | – | 290 |
The diagnosis of CIE is made by excluding the most prevalent and most threatening pathologies, mainly ischemic or hemorrhagic strokes. Another etiology to rule out is posterior reversible encephalopathy syndrome (PRES), which shares pathophysiological factors and whose differentiation is complex (Table 3). Other etiologies to rule out are infections, vasculitis, and venous thrombosis.21
Differences between contrast-induced encephalopathy (CIE) and posterior reversible encephalopathy syndrome (PRES).
| EIC | PRES | |
|---|---|---|
| Clinic | Encephalopathy, focality, cortical blindness (40%) | Encephalopathy, seizures, headache, visual disturbances (33%). |
| Background | Exposure to radiological contrast | Uncontrolled HTN, immunosuppressants, autoimmune diseases. |
| Radiology | Unilateral cortical and/or subarachnoid hyperdensity, CT edema | Bilateral parieto-occipital vasogenic edema. |
| Duration | Resolution 48−72 h | 2−8 days |
| Treatment | Support measures | Blood pressure control, avoid oscillations.Stop medication causing PRESControl of contributing factors (sepsis, preeclampsia, autoimmune diseases) |
| Forecast | Recovery > 90%. | Benign |
CT, computed tomography; Sickness, disease; HTN: arterial hypertension.
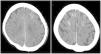
The first recommended test, after a detailed neurological examination, is usually a brain CT without contrast, which could show brain edema plus enhancement of cortical or subarachnoid space of the affected areas,22 although it may occur that radiological signs are absent.20 Particularly the dual energy CT allows to differentiate whether the observed hyperdensities correspond to contrast or hemorrhage.12 The reported cases of patients on HD do not present different radiological signs (Fig. 1). Another useful test is MRI, which is able to demonstrate ischemic lesions, mainly with the DWI sequence through the restriction in diffusion produced by ischemia; the FLAIR sequence identifies the edematous areas22 (Fig. 2). Another test that helps to differentiate, especially from subarachnoid hemorrhage, is the analysis of cerebrospinal fluid (CSF), where the absence of xanthochromia or red blood cells would argue against hemorrhage. Additionally, a higher concentration of contrast in CFS than blood would strongly suggest contrast extravasation.4
Brain computed tomography (CT) scan without contrast of a 63-year-old woman on hemodialysis undergoing third endovascular embolization of an intact anterior communicating artery aneurysm with postprocedural left hemiparesis (having undergone two previous uneventful interventions in the past). CT scan shows cortical hyperdensity in the sulcus of both frontal lobes. Matsubara et al.3 With permission of the authors.
Magnetic resonance imaging (MRI) of an 84-year-old woman with left hemiplegia and right gaze deviation with loss of consciousness after cardiac catheterization. MRI two hours after the procedure shows effacement of sulci at the right parieto-occipital level with respect to the contralateral with cortico-subcortical hyperintensity at that level in T2 and FLAIR, suggestive of cerebral edema and absence of ischemic lesions in DWI. Meng-Ru et al.19 With permission of the authors.
There are no unified diagnostic criteria for CIE. Only the group from Taiwan, Chu et al.13 established diagnostic criteria within the first 24 h of the clinical presentation in patients with previous neurological symptoms (undergoing endovascular thrombectomy after acute ischemic stroke), combining clinical data (increase of four or more points on the National Institutes of Health Stroke Scale [NIHSS], worsening of the Glasgow scale by two or more points, or delayed improvement after thrombectomy not explained by the original ischemic area, recurrent stroke or hemorrhagic transformation) and radiological findings (edematous changes beyond the ischemic area together with contrast enhancement). Nevertheless, these criteria have not been validated in a separate external cohort. There are also no data suggesting different diagnostic criteria in patients with ACKD.
Perhaps the most difficult pathology to discern is PRES, which is based on cerebral vasogenic edema secondary to an alteration of the BBB in the context of pronounced hypertension or major fluctuations in blood pressure, autoimmune diseases, immunosuppression or kidney disease. Neurological symptoms are nonspecific and may overlap with CIE, although the largest series report that encephalopathy, seizures and headache are the most prevalent, in contrast to CIE (Table 3). Radiologically, edema is classically evident in the parieto-occipital lobes of both hemispheres, but involvement of other areas such as the frontal or temporal lobe is not uncommon; the subcortical white matter is also affected by edema which is usually asymmetrical, but almost always it is bilateral.23 Thus, the main aid for the differential diagnosis will be the relevant history on the use of iodinated contrast and the presence of hyperdensities in CT in favor of CIE.
Treatment and preventionThere are no clinical trials comparing different therapeutic strategies for CIE. There is only observational data and clinical experiences in which the therapeutic range goes from mere observation with life support to emergency kidney replacement therapy (KRT).
In the systematic review conducted by Quintas-Neves et al.24 in patients with CIE after CNS angiography with subsequent imaging tests, they found that the most commonly used treatment in addition to support is corticoid therapy (50%), perhaps because of its beneficial effect on cerebral edema in other pathologies, to reduce intracranial pressure; other treatments are intense fluid therapy (37.5%), mannitol (18.8%), antiepileptic drugs (10.4%), used only in cases with seizures, and calcium antagonists (10.4%). However, they found no difference with respect to clinical recovery, concluding that there are no recommendations beyond monitoring and supportive treatment.
In another systematic review on CIE after cardiac catheterization,4 only two clinical cases of patients with CKD on HD (2/52) were reported from 1970 to 2017, in which they were the only cases who received KRT with HD as support with subsequent clinical improvement. Cases have been described also with cerebral angiography of patients on HD undergoing urgent HD after clinical presentation with good evolution.25 Likewise, Matsubara et al.3 described three cases of CIE in patients on chronic HD therapy who presented CIE after cerebral aneurysm embolization, who underwent an HD session the following day with clinical improvement. Therefore, the evidence to date reserves the use of KRT in the form of HD to those patients who are chronically on HD, and there are no data to support the indication for patients with preserved renal function or with ACKD.
There are no studies that directly evaluate preventive strategies. Only secondary conclusions have been drawn from those studies aiming to find out the risk factors for CIE, being the minimization of contrast doses the only advice currently in force,10 as well as the classic recommendation of proper hydration to avoid other related toxicities.
PrognosisThe evolution of this pathology is usually favorable with complete neurological recovery between 24−72 h,10 but some cases have been described with a duration of up to 10 days with subsequent recovery.4 In CIE after cardiac catheterization, a 96% (50/52) recovered and two patients did not recover completely; while in the cases described after CNS angiography24 the recovery was 89.6% (43/48), four with partial improvement and one patient died. The patients on HD all presented complete recovery after the relevant session of KRT.3 There are no data suggesting a worse prognosis or a more torpid evolution of patients with ACKD.
Finally, cases have been reported in the past of fatal CIE demonstrated by autopsy after aortography (3/8) or cardiac angiography (5/8), all cases involved the use of hyperosmolar contrast agents, which are no longer used today. We only found one case of fatal CIE secondary to carotid angiography with iopamidol (low osmolality), with a decrease in the level of consciousness 14 h after the intervention and clinical signs compatible with cerebral herniation with poor subsequent evolution.26 We must emphasize that this is the only case described of an infrequent pathology with more than 90% of complete recovery.
FinancingThe authors declare the absence of funding for this research.
Conflict of interestThe authors declare no conflict of interest for this research.