Inhibitors of sodium-glucose co-transporter type 2 (iSGLT2) are newly developed drugs to improve glycemic control of patients with DM2. They act by blocking the absorption of glucose in the proximal tubule, increasing the renal excretion of glucose that is associated to natriuresis and activation of the tubuloglomerular feedback.1 Although an increase in the bone resorption of phosphorus associated with the use of these drugs has been demonstrated, no analytical alterations have been observed in bone-mineral metabolism to date.2
We present a case of hypercalcemia related to the use of dapagliflozin in a 63-year-old woman diagnosed with arterial hypertension, DM2 and dyslipidemia. She was referred for study of albuminuria and was on olmesartan/amlodipine/hydrochlorothiazide 20/5/12.5mg, atorvastatin 40mg, atenolol 25mg, allopurinol 100mg and metformin 850mg/12h.
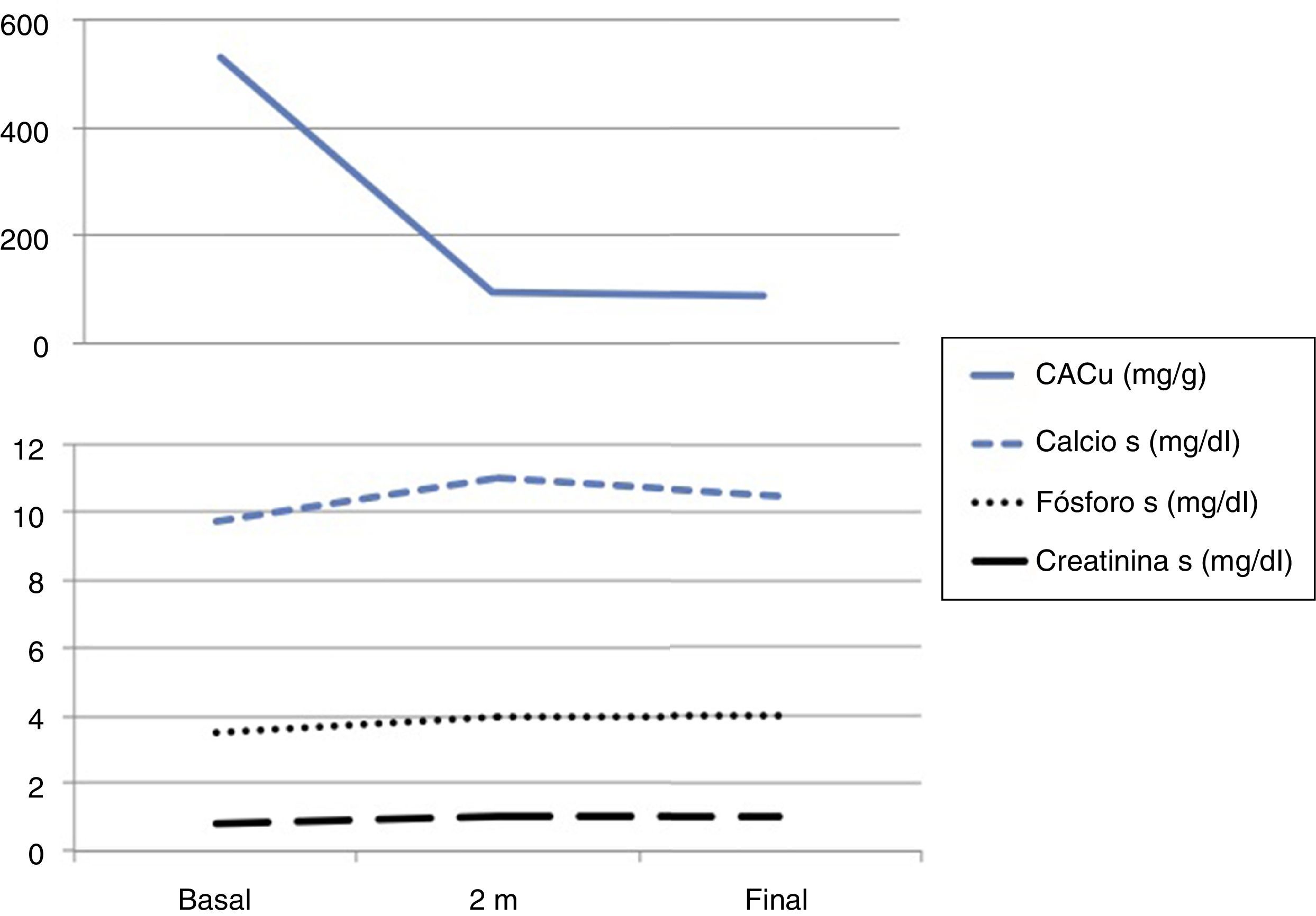
The physical examination was anodyne and the complementary explorations performed showed: Cr 0.8.8mg/dl, estimated glomerular filtration rate by CKD-EPI 78ml/min, calcium 9.7mg/dl, phosphorus 3.5mg/dl, PTH 32pg/ml, 25 (OH) -vitamin D 52ng/dl, glycemia 150mg/dl and HbA1c 7%. The Albu/Cru ratio in urine was 531mg/g and the sediment urine was bland. Ultrasound showed normal and symmetric kidneys. Eye fundi did not show diabetic or hypertensive retinopathy.
The patient was diagnosed of CKD G2A3 probably associated with diabetes. After a failed attempt to increase the dose of olmesartan (not tolerated by orthostatic hypotension and hyperkalaemia), dapagliflozin 5mg/12h was associated to the treatment
After 2 months the patient was asymptomatic with good blood pressure control (120/70mmHg) and in the laboratory test presented serum calcium 11.0mg/dl, phosphorus 4.0mg/dl, PTH 40pg/dl, Cr 1.0mg/dl (eGFR by CKD-EPI of 60ml/min) and potassium 5.7mmol/dl (Fig. 1). Re-interrogated recognized the milk intake supplemented with calcium on a regular basis. Hydrochlorothiazide was changed to torasemide, 5mg/24h, it was recommended to increase the water intake and to restrict dairy products and the serum calcium decreased to 10.4mg/dl (Fig. 1). Renal function remained stable after the slight drop in the eGFR (Fig. 1) with a significant decrease in albuminuria and good metabolic control.
The patients was diagnosed of moderate hypercalcemia related to the used of SGLT2 inhibitors that resolved after removal of other factors favoring hypercalcemia which allowed to maintain the medication.
We describe a case of hypercalcemia associated to dapagliflozin in a patient with other risk factors to develop hypercalcemia: Thiazides and high calcium intake.
The iSGLT2 have effects on calcium-phosphorus metabolism because they induce increased tubular reabsorption of phosphorus,1 but there are no cases reported in the literature of hypercalcemia attributable to dapagliflozin to date. In preclinical studies, some iSGLT2 inhibitors induced mild increases in calcemia, an effect attributed to a partial inhibition of intestinal SGLT1 receptors. The malabsorption of carbohydrates causes a decrease in intestinal pH and an increase in intestinal calcium absorption.3 However, subsequent safety studies have not observed significant electrolyte alterations in relation to these drugs.4 Only one case of severe hypercalcemia has been described in relation to the use of canagliflocin in a patient with high oral calcium intake, severe volume depletion and diabetic ketoacidosis.3 In the case presented here, the occurrence of hypercalcemia is probably due to the concurrence of several factors: food intake supplemented with calcium, decreased renal excretion due to thiazides and increased intestinal absorption induced by dapagliflozin.
This is the first case described of hypercalcemia associated with the use of dapagliflozin. In view of the data published in the literature, this should be a rare side effect that requires the concurrence of other factors that induce hypercalcemia. The severity of the condition was mild and did not require the suspension of the drug.
Please cite this article as: Marques Vidas M, Dura Gurpide B, Rubio E, Huerta A, Portolés Pérez J. Hipercalcemia inducida por dapagliflozina. Nefrologia. 2018;38:336–337.