Hyperkalemia (HK) is an electrolyte disturbance in the concentration of potassium ions (K+), whose risk increases in patients with chronic kidney disease (CKD) or heart failure (HF) and/or in patients being treated with renin–angiotensin–aldosterone system inhibitors (RAASi). The new oral K+ chelators offer a safe and effective treatment to maintain normokalemia in these patients. The objective of the analysis is to estimate the cost-effectiveness of sodium zirconium cyclosilicate (SZC) for the treatment of chronic HK in patients with CKD or HF versus standard treatment (calcium polystyrene sulfonate and lifestyle modifications) from the perspective of the Spanish National Health System.
Materials and methodsTwo microsimulation models reflecting the natural history of CKD and HF were used. In both models, K+ levels were simulated individually. Based on efficacy (reduction of K+ levels), quality of life of patients (utilities according to health states, and disutilities of events derived from each pathology and adverse events [AEs] of treatment) and costs considered (cost of treatment for HK, of RAASi treatment and its modification, health states, management of events derived from each pathology, HK episodes, and AEs treatment) (, 2022), clinical benefit (quality-adjusted life years [QALYs]) and cost results were obtained. A time horizon of the patient’s lifetime was used and a discount rate of 3% was applied for costs and outcomes.
ResultsSZC is a more effective option in both pathologies, with a difference in QALYs of 0.476 in CKD and 0.978 in HF compared to standard treatment, and it represents an incremental cost of 3,616 and 14,749, respectively, obtaining an incremental cost-utility ratio of 7,605/QALY in CKD and 15,078/QALY in HF.
ConclusionsSZC is a cost-effective alternative for the treatment of HK in patients with CKD or HF, taking into account the reference efficiency values commonly used in Spain.
La hiperpotasemia (HK) es una alteración electrolítica en la concentración de iones potasio (K+), cuyo riesgo aumenta en pacientes con enfermedad renal crónica (ERC) o insuficiencia cardiaca (IC) y/o en pacientes en tratamiento con inhibidores del sistema renina-angiotensina-aldosterona (iSRAA). Los nuevos quelantes orales de K+ ofrecen un tratamiento seguro y eficaz para mantener la normopotasemia en estos pacientes. El objetivo del análisis es estimar el coste-efectividad del ciclosilicato de sodio y zirconio (CSZ) para el tratamiento de la HK crónica en pacientes con ERC o IC frente al tratamiento estándar (poliestireno sulfonato cálcico y modificaciones del estilo de vida) desde la perspectiva del Sistema Nacional de Salud (SNS) español.
Materiales y métodosSe utilizaron dos modelos de microsimulación que reflejan la historia natural de la ERC y de la IC. En ambos modelos se realizó una simulación de forma individual de los niveles de K+. En base a la eficacia (reducción de los niveles de K+), la calidad de vida de los pacientes (utilidades según estado de salud, y disutilidades de los eventos derivados de cada patología y los eventos adversos [EA] del tratamiento) y a los costes contemplados (coste del tratamiento para la HK, del tratamiento con iSRAA y su modificación, de los estados de salud, del manejo de los eventos derivados de cada patología, de los episodios de HK, y de los EA del tratamiento) (, 2022), se obtuvieron resultados de beneficio clínico (años de vida ajustados por calidad [AVAC]) y costes. Se empleó un horizonte temporal de toda la vida del paciente y se aplicó una tasa de descuento del 3% para costes y resultados.
ResultadosEl CSZ resulta una opción más efectiva en ambas patologías, con una diferencia de AVAC de 0,476 en ERC, y de 0,978 en IC frente al tratamiento estándar, y supone un coste incremental de 3.616 y 14.749 , respectivamente, obteniéndose un ratio coste-utilidad incremental (RCUI) de 7.605 /AVAC en ERC y 15.078 /AVAC en IC.
ConclusionesEl CSZ es una alternativa con una buena relación coste-efectividad para el tratamiento de la HK en pacientes con ERC o IC, teniendo en cuenta los valores de eficiencia de referencia empleados habitualmente en España.
Hyperkalaemia (HK) is an electrolyte disturbance defined as a serum potassium ion (K+) concentration >5.0 mEq/l or >5.5 mEq/l.1 The risk of developing HK is increased in patients with chronic kidney disease (CKD) or heart failure (HF),2,3 as the kidney is the main organ regulating K+ homeostasis. Renin–angiotensin–aldosterone system inhibitors (RAASi), which are beneficial in these two conditions, also reduce renal excretion of K+.3,4 This is why, in both conditions, it is particularly important to maintain adequate K+ levels while maintaining optimal treatment with RAASi.4,5 According to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, HK can be classified as mild (5.0–5.9 mEq/l) or moderate/severe (≥6.0 mEq/l) based on serum K+ concentration.6 Mild HK is usually asymptomatic. However, in more severe cases, HK is associated with high morbidity and mortality rates due to factors such as the risk of triggering severe cardiac arrhythmias, which can be fatal.2,3,7
The aim of HK treatment is to reduce serum K+.8 To date, management has been based on dietary recommendations, reduction or discontinuation of drugs such as RAASi, or the use of cation-exchange resins such as calcium polystyrene sulfonate or sodium polystyrene sulfonate.8–10 However, the use of these resins is associated with occasionally severe gastrointestinal adverse effects and poor tolerability, which can result in poor adherence to treatment.9,11
The development and approval of sodium zirconium cyclosilicate (SZC), an inorganic compound that binds to K+ in the gastrointestinal tract, provides an alternative to the classic therapies.12 Clinical trials have shown that SZC is effective in reducing K+ levels to normal levels within 48 h,13,14 even in patients with CKD or cardiovascular disease receiving RAASi, without the need to lower the dose of the inhibitor or temporarily discontinue treatment,14 and its effects are sustained over the long term.15,16 In addition, SZC has shown a favourable safety profile, with mild-to-moderate adverse events (AE) that are manageable and do not require discontinuation of the treatment.16
The aim of this study was to perform a cost-effectiveness analysis of SZC vs standard treatment (calcium polystyrene sulfonate and lifestyle change) for chronic HK in patients with either CKD or HF, from the perspective of the Spanish National Health System (SNS).
MethodsTwo patient-level simulation models were used, reflecting the natural history of how a typical patient with CKD or HF, respectively, would progress, in terms of serum K+ levels and compliance with RAASi therapy. For both conditions, both the clinical consequences (life-years gained [LYG], quality-adjusted life-years [QALY]) and economic consequences (costs of treatment [HK and RAASi], events associated with each condition and management of AE related to HK treatment) of SZC treatment compared to standard treatment in Spain (calcium polystyrene sulfonate and lifestyle change) were estimated from the perspective of the SNS. Based on the information generated, incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR) were calculated for each of the simulated cases. The structure of the models, as well as their parameters and main assumptions, were agreed and validated by the authors of this article (two nephrology specialists, two cardiology specialists and one health economist).
In both cases, a lifetime time horizon and a cycle length of four weeks (the period after which costs and health effects were assessed) were used, with the exception of the first period, where shorter cycles were considered based on the design of the SZC reference trials,13,15,16 to reflect changes in K+ levels during the HK event (acute phase). As recommended in the Spanish reference guidelines, a discount rate of 3% was applied for costs and outcomes.17
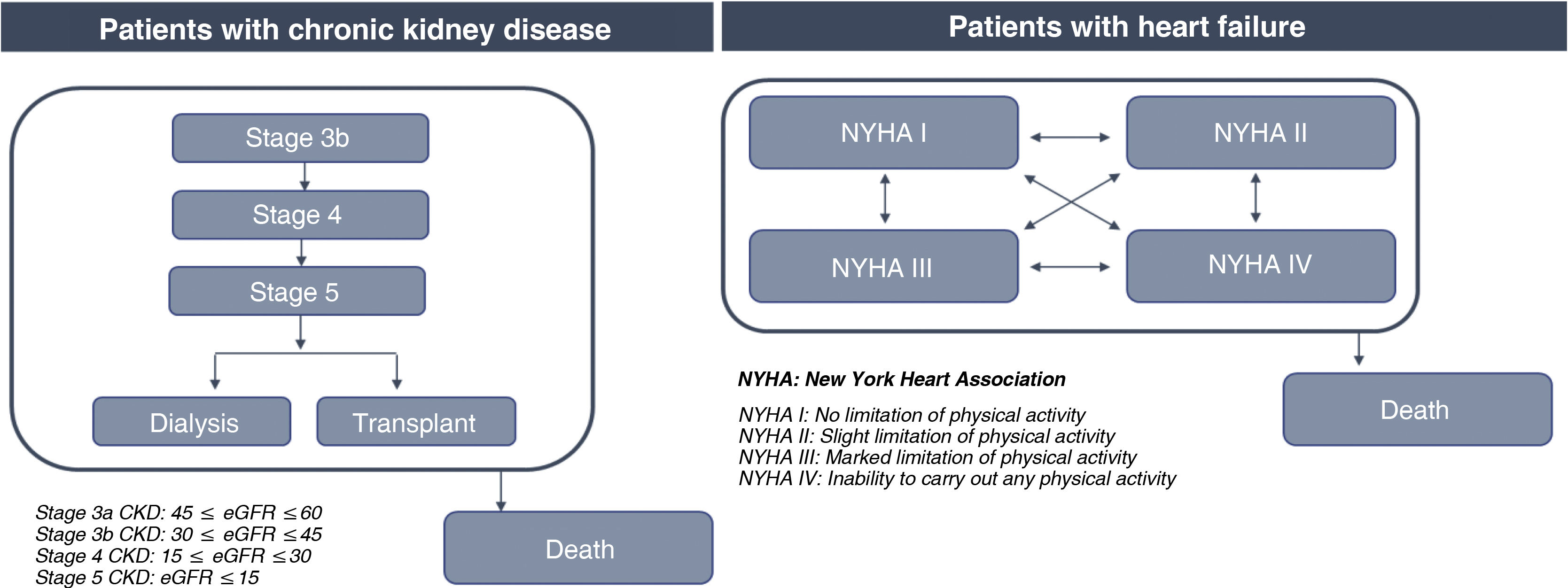
In the CKD model, patients with CKD stage 3b with an HK event enter the model receiving SZC or standard treatment. Their disease may progress to more advanced stages based on an annual decline in estimated glomerular filtration rate (eGFR) dependent on RAASi (3b-5), after which they will receive renal replacement therapy (RRT: dialysis or transplantation) (Fig. 1).
In the HF model, patients with HF who have an HK event enter the model receiving SZC or standard treatment, and may be in any of the four stages of the New York Heart Association functional classification (NYHA I–IV), through which they transit (Fig. 1).
In addition, K+ levels were individually simulated based on the efficacy of the treatment received (each patient will have a unique K+ level trajectory). Likewise, after an HK event, consideration was given to the possible reduction (from optimal to suboptimal dosing) or discontinuation of RAASi therapy. A percentage of these patients will return to optimal dosing after the HK event, but if they experience a new HK event leading to dose reduction or discontinuation of RAASi therapy after returning to optimal dosing, they will continue in this state until the end of the simulation (Appendix A, Tables S1 and S2).
In addition, patients could have major adverse cardiovascular events (MACE) and hospitalisations depending on their health status and K+ levels. In both models it was considered that patients could move to “death” status at any time and from any health status.
This study adheres to the standards recommended in the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).18
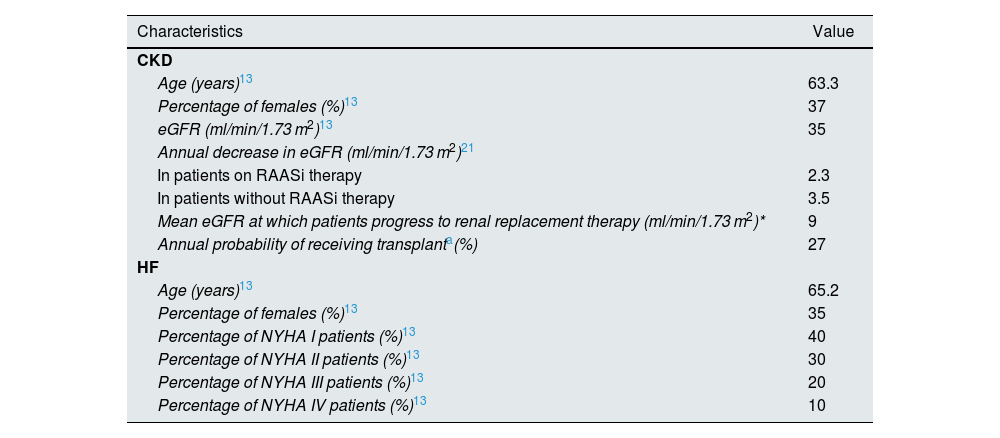
PopulationThe base case was simulated with a cohort of 30,000 patients with CKD and a cohort of 30,000 patients with HF who had experienced an HK event (serum K+ levels ≥5.5 mEq/l). Patient characteristics for the two cohorts were extracted from both the HARMONIZE study (ZS-004; NCT02088073)13 and the Clinical Practice Research Datalink (CPRD)19 database (Table 1, Appendix A Table S3).
Clinical parameters of the model.
| Characteristics | Value |
|---|---|
| CKD | |
| Age (years)13 | 63.3 |
| Percentage of females (%)13 | 37 |
| eGFR (ml/min/1.73 m2)13 | 35 |
| Annual decrease in eGFR (ml/min/1.73 m2)21 | |
| In patients on RAASi therapy | 2.3 |
| In patients without RAASi therapy | 3.5 |
| Mean eGFR at which patients progress to renal replacement therapy (ml/min/1.73 m2)* | 9 |
| Annual probability of receiving transplanta(%) | 27 |
| HF | |
| Age (years)13 | 65.2 |
| Percentage of females (%)13 | 35 |
| Percentage of NYHA I patients (%)13 | 40 |
| Percentage of NYHA II patients (%)13 | 30 |
| Percentage of NYHA III patients (%)13 | 20 |
| Percentage of NYHA IV patients (%)13 | 10 |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HF: heart failure; NYHA: New York Heart Association; RAASi: renin–angiotensin–aldosterone system inhibitors.
In patients with CKD it was considered a baseline eGFR of 35 ml/min/1.73 m2, which decreased annually in accordance with their RAASi therapy. When a value of 9.00 ml/min/1.73 m2 was reached, patients were switched to dialysis or transplantation on the basis of the annual probability of transplantation. In patients with HF, the transition between the stages of the NYHA classification was modelled based on the monthly probability of moving from one stage to another (Table 1).20
Simulation of individual serum K+ levels was based on data from the HARMONIZE study13 for the first 28 days and from the study by Spinowitz et al. (ZS-005)16 for the remainder of the simulation. After the initial episode of HK, patients could experience new episodes of HK with each cycle (K+ levels ≥5.5 mEq/l).
In patients with CKD, the annual probability of suffering a MACE or hospitalisation was calculated from their annual rate based on health status (which was extracted from the study by Go et al., based on the California Kaiser Permanente registry where 1,120,295 patients with CKD at different stages were studied),22 and adjusted for the patient’s K+ levels,23 eGFR (only for hospitalisations),23 whether they were receiving RAASi therapy24 and whether their dose was optimal (assuming that the efficacy of RAASi decreases to 50% at suboptimal doses). In patients with HF, the annual probability of MACE was estimated using a risk equation based on CPRD20 data (Appendix A, Table S4), which was adjusted for patient levels of K+, assuming the same data as in those with CKD in the absence of specific data.23 The monthly probability of hospitalisation was calculated using the incidence extracted from the CPRD19 based on RAASi use and K+ levels (Appendix A, Table S5).
In both conditions, an indefinite duration of HK treatment was assumed when patients received SZC, except in CKD patients who progressed to end-stage disease and started receiving RRT. However, in both CKD and HF, the annual probability of discontinuation of SZC treatment was considered to be 26.4%.16,25 For standard treatment, since it is based on lifestyle change and treatment with calcium polystyrene sulfonate during the acute phase (three days), it was assumed that no patient discontinued treatment.
MortalityFor CKD patients, the risk of death was based on the patient’s health status, taken from the above-mentioned study by Go et al.,22 which was adjusted for levels of K+23 and use of RAASi24 (assuming a 50% reduction in RAASi efficacy at suboptimal doses). For HF, the risk of death was calculated using a prediction model (The Seattle Heart Failure) based on the publication by Levy et al.26 (Appendix A, Table S6), which was adjusted for levels of K+.27 In both cases, if the resulting risk was lower than the mortality risk of the general Spanish population, thus, the latter was used.28
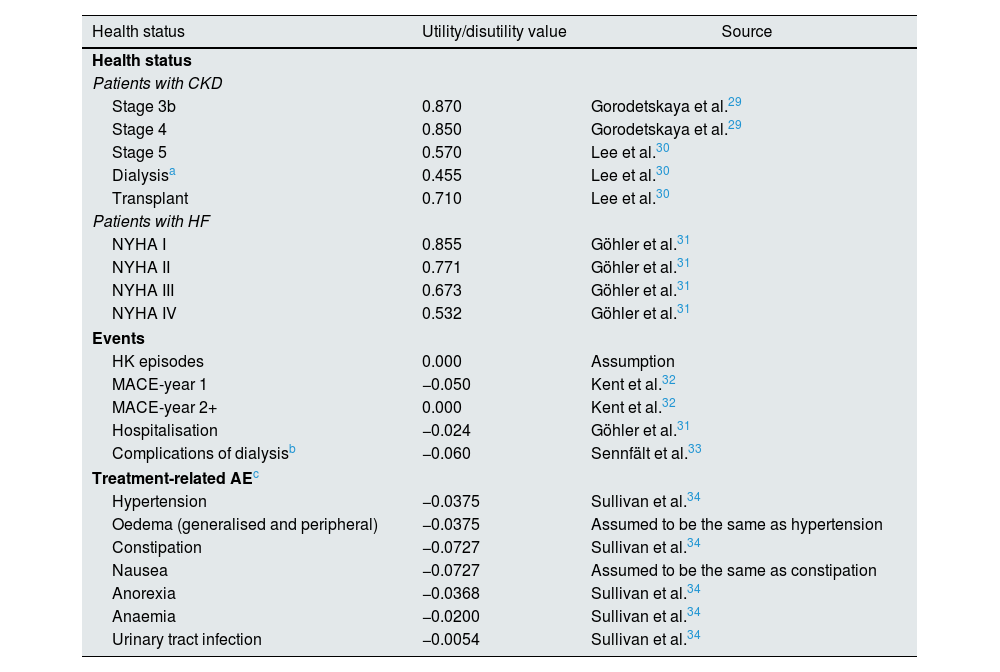
Utility valuesTo incorporate the impact on health status and quality of life of CKD and HF, the episodes HK and treatment-related AE, there were taken into account the utility values associated with each health status, as well as the disutility values associated with HK episodes, MACE, hospitalisations, dialysis complications and treatment-related AE (Table 2).
Utility and disutility values considered in the model.
| Health status | Utility/disutility value | Source |
|---|---|---|
| Health status | ||
| Patients with CKD | ||
| Stage 3b | 0.870 | Gorodetskaya et al.29 |
| Stage 4 | 0.850 | Gorodetskaya et al.29 |
| Stage 5 | 0.570 | Lee et al.30 |
| Dialysisa | 0.455 | Lee et al.30 |
| Transplant | 0.710 | Lee et al.30 |
| Patients with HF | ||
| NYHA I | 0.855 | Göhler et al.31 |
| NYHA II | 0.771 | Göhler et al.31 |
| NYHA III | 0.673 | Göhler et al.31 |
| NYHA IV | 0.532 | Göhler et al.31 |
| Events | ||
| HK episodes | 0.000 | Assumption |
| MACE-year 1 | −0.050 | Kent et al.32 |
| MACE-year 2+ | 0.000 | Kent et al.32 |
| Hospitalisation | −0.024 | Göhler et al.31 |
| Complications of dialysisb | −0.060 | Sennfält et al.33 |
| Treatment-related AEc | ||
| Hypertension | −0.0375 | Sullivan et al.34 |
| Oedema (generalised and peripheral) | −0.0375 | Assumed to be the same as hypertension |
| Constipation | −0.0727 | Sullivan et al.34 |
| Nausea | −0.0727 | Assumed to be the same as constipation |
| Anorexia | −0.0368 | Sullivan et al.34 |
| Anaemia | −0.0200 | Sullivan et al.34 |
| Urinary tract infection | −0.0054 | Sullivan et al.34 |
AE: adverse events; CKD: chronic kidney disease; HF: heart failure; HK: hyperkalaemia; MACE: major adverse cardiovascular events; NYHA: New York Heart Association.
Calculation: utility of haemodialysis (0.440) and peritoneal dialysis (0.530) weighted by proportion of use (83.3% and 16.7%, respectively) extracted from the Spanish registry of renal patients.35.
For patients starting RRT, only specific utilities and dialysis disutility are taken into account (events prior to RRT are not taken into account).
The model takes into account events occurring with an incidence ≥5% for the alternatives compared, drawn from study ZS-00536 for SZC and from Nasir et al.37 for standard treatment (data are taken for patients receiving calcium polystyrene sulfonate treatment in the acute phase of HK). Urinary tract infection is not taken into account, on the assumption of the experts.
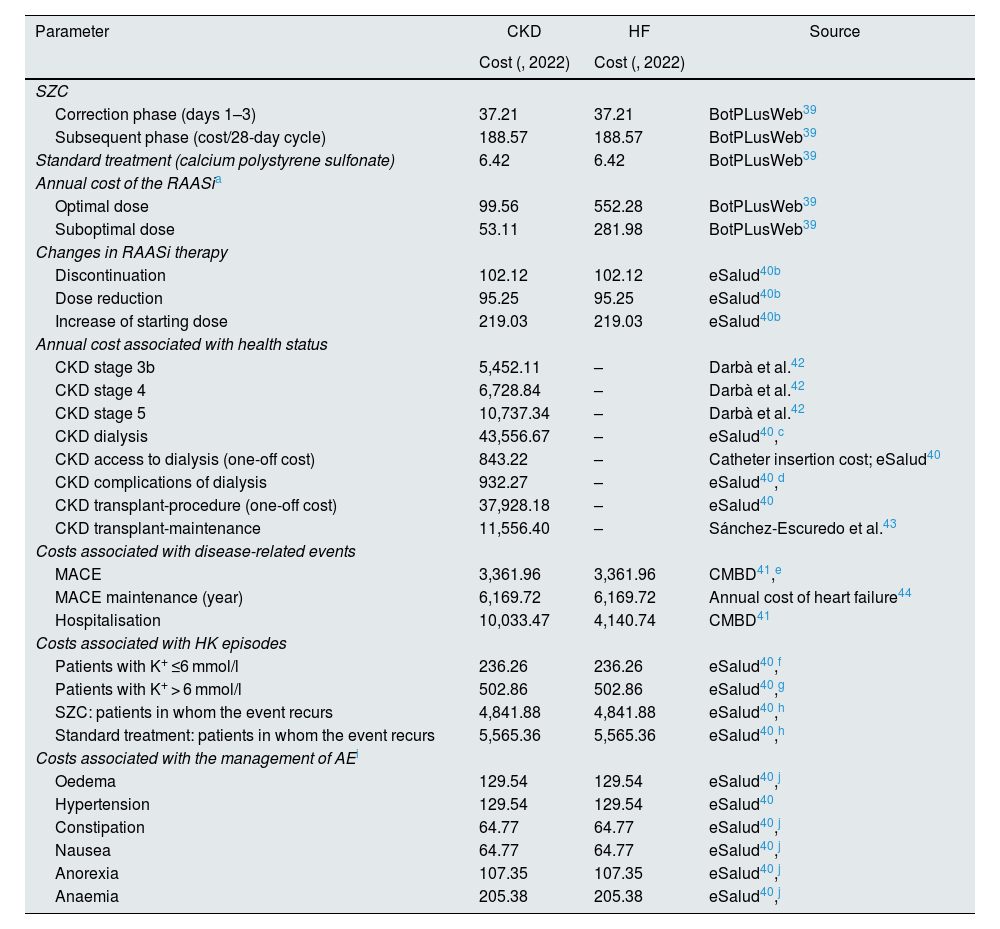
Direct costs associated with drug treatments, modifications in RAASi therapy, costs associated with disease and event management, those associated with HK episodes (depending on their severity, defined as low: 5.5 ≤ K+ ≤ 6.0 mEq/l, or medium/high: K+ > 6.0 mEq/l) and treatment-related AE were taken into account. For drug costs, we used the costs of the treatment for HK, SZC and calcium sulfonate polystyrene, and of RAASi therapy (the cost of which depended on whether the dose was optimal or suboptimal; considerations for the calculation of the annual cost are shown in Appendix A, Table S7). For cost of SZC, we calculated the cost/day in the K+ correction phase where, according to the authors’ estimation, on day one, 10.0% of patients use 5 g/day, 25.0% 10 g/day and 65.0% 10 g/3 times a day; on day two, 10.0% use 5 g/day, 73.3% 10 g/day and 16.7% 10 g/3 times a day; and on day three, 20.0% use 5 g/day, 76.7% 10 g/day and 3.3% 10 g/3 times a day. We also calculated the cost/day of the subsequent phase, taking as a reference the percentage of use of each dose in the SZC extension study36 (where 0.9% use 5 g/2 days, 61.7% 5 g/day and 37.4% 10 g/day). For the calculation of the cost of the calcium polystyrene sulfonate dose, we considered the cost of the drug for first phase of correction of the HK (days 1–3), assuming a defined daily dose of 45 g/day.38 In relation to the costs of changes in RAASi therapy, the costs of discontinuing treatment, reducing the dose and increasing the starting dose were taken into account. With regard to the costs originated from disease management, the costs associated with the health status of each of the diseases were taken into account, which, in the case of HF, were conservatively assumed to be event driven, so that no direct cost was associated with each NYHA functional class. The costs of managing events arising from CKD and HF (MACE and hospitalisations) and HK episodes (the cost of which depended on their severity) were included. Lastly, we considered the cost of the HK treatment-related AE (SZC and calcium polystyrene sulfonate).
The drug costs (price of sale of the pharmaceutical company [wholesale price] excluding VAT) were extracted from the BotPLusWeb database.39 Unit costs were obtained from national databases.40,41 The base or reference year for the cost assessment was 2022 (Table 3).
Costs considered in the model.
| Parameter | CKD | HF | Source |
|---|---|---|---|
| Cost (, 2022) | Cost (, 2022) | ||
| SZC | |||
| Correction phase (days 1–3) | 37.21 | 37.21 | BotPLusWeb39 |
| Subsequent phase (cost/28-day cycle) | 188.57 | 188.57 | BotPLusWeb39 |
| Standard treatment (calcium polystyrene sulfonate) | 6.42 | 6.42 | BotPLusWeb39 |
| Annual cost of the RAASia | |||
| Optimal dose | 99.56 | 552.28 | BotPLusWeb39 |
| Suboptimal dose | 53.11 | 281.98 | BotPLusWeb39 |
| Changes in RAASi therapy | |||
| Discontinuation | 102.12 | 102.12 | eSalud40b |
| Dose reduction | 95.25 | 95.25 | eSalud40b |
| Increase of starting dose | 219.03 | 219.03 | eSalud40b |
| Annual cost associated with health status | |||
| CKD stage 3b | 5,452.11 | – | Darbà et al.42 |
| CKD stage 4 | 6,728.84 | – | Darbà et al.42 |
| CKD stage 5 | 10,737.34 | – | Darbà et al.42 |
| CKD dialysis | 43,556.67 | – | eSalud40,c |
| CKD access to dialysis (one-off cost) | 843.22 | – | Catheter insertion cost; eSalud40 |
| CKD complications of dialysis | 932.27 | – | eSalud40,d |
| CKD transplant-procedure (one-off cost) | 37,928.18 | – | eSalud40 |
| CKD transplant-maintenance | 11,556.40 | – | Sánchez-Escuredo et al.43 |
| Costs associated with disease-related events | |||
| MACE | 3,361.96 | 3,361.96 | CMBD41,e |
| MACE maintenance (year) | 6,169.72 | 6,169.72 | Annual cost of heart failure44 |
| Hospitalisation | 10,033.47 | 4,140.74 | CMBD41 |
| Costs associated with HK episodes | |||
| Patients with K+ ≤6 mmol/l | 236.26 | 236.26 | eSalud40,f |
| Patients with K+ > 6 mmol/l | 502.86 | 502.86 | eSalud40,g |
| SZC: patients in whom the event recurs | 4,841.88 | 4,841.88 | eSalud40,h |
| Standard treatment: patients in whom the event recurs | 5,565.36 | 5,565.36 | eSalud40,h |
| Costs associated with the management of AEi | |||
| Oedema | 129.54 | 129.54 | eSalud40,j |
| Hypertension | 129.54 | 129.54 | eSalud40 |
| Constipation | 64.77 | 64.77 | eSalud40,j |
| Nausea | 64.77 | 64.77 | eSalud40,j |
| Anorexia | 107.35 | 107.35 | eSalud40,j |
| Anaemia | 205.38 | 205.38 | eSalud40,j |
AE: adverse events; CKD: chronic kidney disease; HF: heart failure; MACE: major adverse cardiovascular events; PVL: precio venta laboratorio [wholesale price] (for SZC, the notified price is used, and for calcium polystyrene sulfonate, the reference price); RAASi: renin-angiotensin-aldosterone system inhibitors; SZC: sodium zirconium cyclosilicate.
The considerations taken into account for calculating the annual cost of RAASi therapy are shown in Appendix A, Table S7.
Discontinuation and dose reduction: laboratory test (biochemistry, 100%), one visit to primary care (53.3%) and one visit to a specialist (46.7%). Management of side effects following discontinuation of the dose: an additional visit to Primary Care and additional laboratory test (20%). Management of possible side effects of dose reduction: one additional visit to Primary Care and additional laboratory test (10%); increase in starting dose: two laboratory tests (biochemistry, 100%), two visits to Primary Care (26.7%), and two visits to a specialist (73.3%). Management of possible side effects of dose increase: one additional visit to Primary Care and one additional laboratory test (20%), and a visit to Accident and Emergency (3%).
Weighted cost of haemodialysis and peritoneal dialysis35: haemodialysis (83.3%), peritoneal dialysis (16.7%). Cost of haemodialysis 45,084.00: 289.00/session (eSalud40) in three weekly sessions (Arieta45). Cost of peritoneal dialysis 36,406.00: training 144.00/session and 97.00/continued session (eSalud40) in seven training sessions for two weeks and one session per day after training (Arieta45).
Extra haemodialysis per week (5%); five days of hospitalisation for fever related to venous access (5%).
One electrocardiogram (80%); 1.7 urea and electrolyte tests (97%); 1.7 Primary Care visits (73%); two outpatient visits (27%).
26.7% are hospitalised (hospitalisation lasting 4.5 days [25%], two electrocardiograms and four urea and electrolyte tests [100%]); and 73.3% have outpatient visits (2.3 outpatient visits [47%]), one electrocardiogram (60%); 2.3 urea and electrolyte tests (73%); one ambulance transport (10%); one Accident and Emergency visit (15%); one nephrology visit (35%).
Two electrocardiograms, five urea and electrolyte tests and five glucose tests (100%); SZC treatment: hospitalisation lasting six days; standard treatment: hospitalisation lasting seven days.
The model takes into account events occurring with an incidence ≥5% for the alternatives compared, drawn from study ZS-00536 for SZC and from Nasir et al.37 for standard treatment (data are taken for patients receiving calcium polystyrene sulfonate treatment in the acute phase of HK). Urinary tract infection is not taken into account, on the assumption of the experts.
In order to assess the robustness of the models and determine the impact of parameter uncertainty on the ICUR, an univariate deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA) were performed. Through the DSA, the main parameters of the model were varied individually (to the upper and lower limits of their confidence interval (95% CI), or ±10% from baseline). The results were plotted on a tornado diagram. The PSA was carried out through a Monte-Carlo simulation of 1000 simulations, whereby variations were randomly assigned to each of the parameters according to their probability distribution. The results were plotted on a scatter plot of the cost-effectiveness plane. Based on existing Spanish literature, a willingness-to-pay threshold of 25,000 per QALY gained was assumed as baseline.46
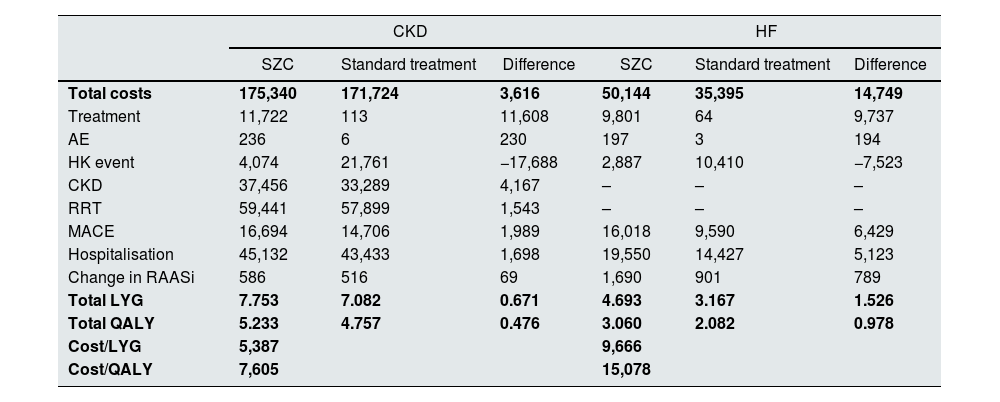
ResultsBase caseIn CKD patients, the results showed a lifetime cost of 175,340 for SZC and 171,724 for the standard treatment (calcium polystyrene sulfonate). The LYG and QALY with SZC were 7.753 and 5.233, respectively, while with standard treatment they were 7.082 and 4.757, respectively, with an estimated ICER of 5,387 and an ICUR of 7,605.
In HF patients, the results showed a cost of 50,144 for SZC and 35,395 for standard treatment. The LYG and QALY with SZC were 4.693 and 3.060, respectively, while with standard treatment they were 3.167 and 2.082, respectively, with an estimated ICER of 9,666 and an ICUR of 15,078 (Table 4).
Base case results.
| CKD | HF | |||||
|---|---|---|---|---|---|---|
| SZC | Standard treatment | Difference | SZC | Standard treatment | Difference | |
| Total costs | 175,340 | 171,724 | 3,616 | 50,144 | 35,395 | 14,749 |
| Treatment | 11,722 | 113 | 11,608 | 9,801 | 64 | 9,737 |
| AE | 236 | 6 | 230 | 197 | 3 | 194 |
| HK event | 4,074 | 21,761 | −17,688 | 2,887 | 10,410 | −7,523 |
| CKD | 37,456 | 33,289 | 4,167 | – | – | – |
| RRT | 59,441 | 57,899 | 1,543 | – | – | – |
| MACE | 16,694 | 14,706 | 1,989 | 16,018 | 9,590 | 6,429 |
| Hospitalisation | 45,132 | 43,433 | 1,698 | 19,550 | 14,427 | 5,123 |
| Change in RAASi | 586 | 516 | 69 | 1,690 | 901 | 789 |
| Total LYG | 7.753 | 7.082 | 0.671 | 4.693 | 3.167 | 1.526 |
| Total QALY | 5.233 | 4.757 | 0.476 | 3.060 | 2.082 | 0.978 |
| Cost/LYG | 5,387 | 9,666 | ||||
| Cost/QALY | 7,605 | 15,078 | ||||
AE: adverse events; CKD: chronic kidney disease; HF: heart failure; HK: hyperkalaemia; LYG: life-years gained; MACE: major adverse cardiovascular events; QALY: quality-adjusted life-years; RAASi: renin–angiotensin–aldosterone system inhibitors; RRT: renal replacement therapy; SZC: sodium zirconium cyclosilicate.
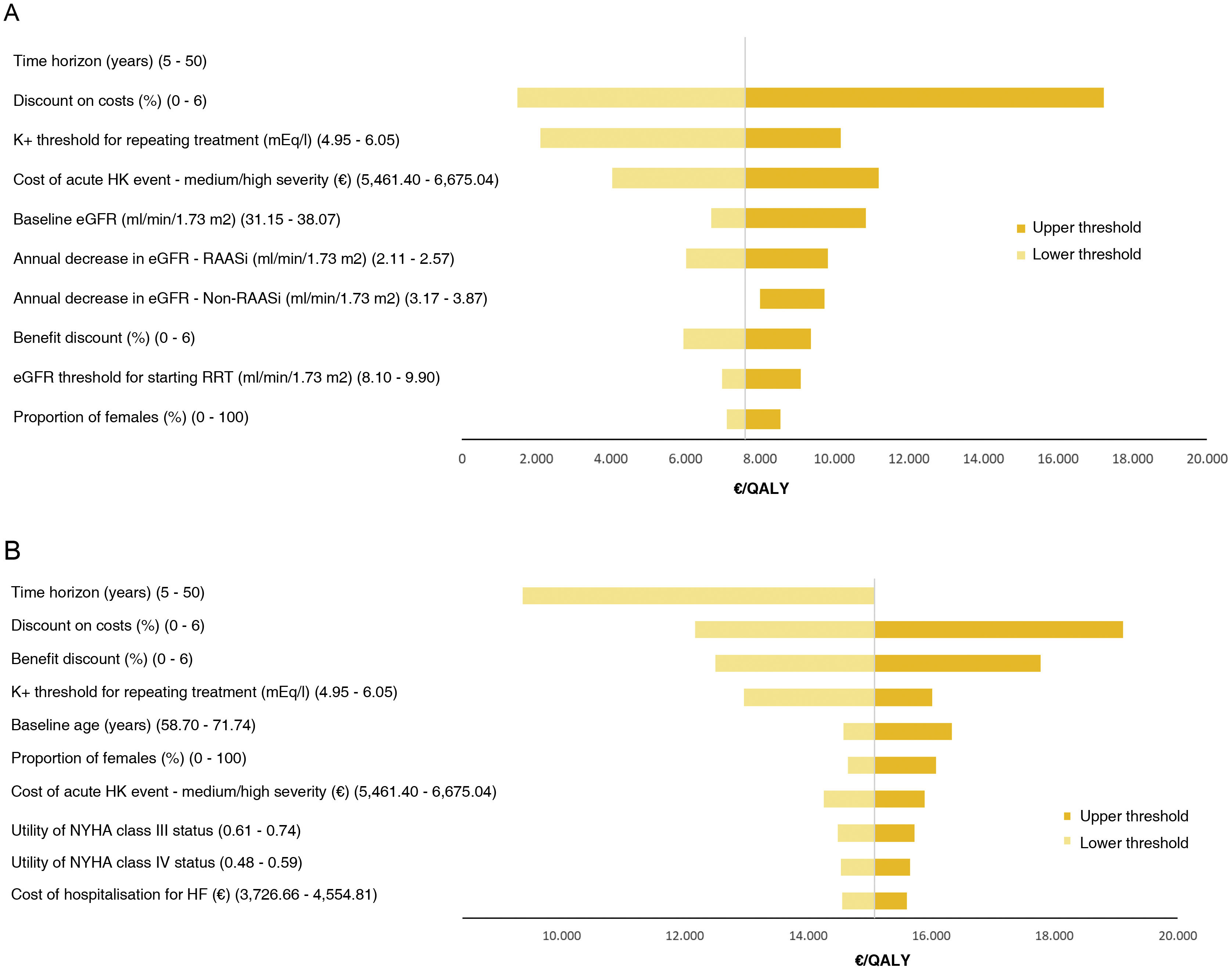
In both CKD and HF, the parameter with the greatest influence on the ICUR was the time horizon considered. For CKD, considering a five-year time horizon would make SZC a dominant option, and a 50-year time horizon would increase the ICUR by 5. The second most influential parameter was the discount applied to costs. However, in all cases SZC is a cost-effective option taking into account a cost-effectiveness threshold of 25,000/QALY (Fig. 2).
DSA.
A: DSA for CKD. The results of varying the time horizon to its lower threshold are not shown, as SZC is in this case a dominant option (less costly and more effective); when varying it to its upper threshold, the ICUR is 7,610, so the line is not visible as it only differs from the base case by 5; B: DSA for HF.
CKD: chronic kidney disease; DSA: deterministic sensitivity analysis; eGFR: estimated glomerular filtration rate; HF: heart failure; HK: hyperkalaemia; ICUR, incremental cost-utility ratio; NYHA: New York Heart Association; RAASi, renin–angiotensin–aldosterone system inhibitors; RRT: renal replacement therapy; SZC, sodium zirconium cyclosilicate.
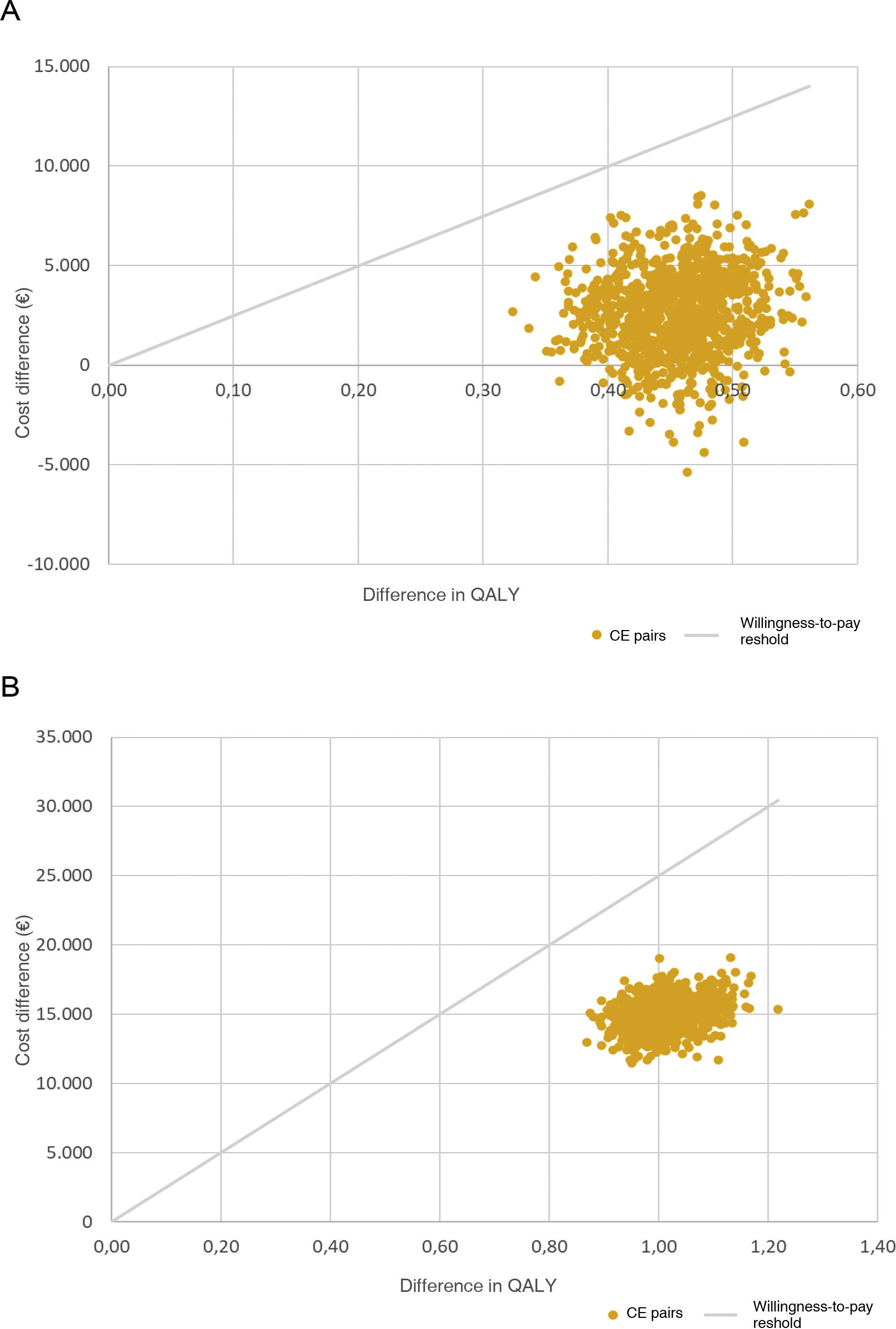
In terms of the probabilistic analysis, for CKD, SZC was a cost-effective option in 90.4% of the simulations, and in the remaining 9.6%, a dominant option (more effective and less costly alternative) vs standard treatment. For HF, SZC proved to be a cost-effective alternative in 100% of the simulations (Fig. 3).
PSA: cost-effectiveness plane.
A: PSA for CKD; B: PSA for HF.
Willingness-to-pay threshold: the maximum amount considered appropriate to invest per unit of health effectiveness in the healthcare system.
CE: cost-effectiveness; CKD: chronic kidney disease; HF: heart failure; PSA: probabilistic sensitivity analysis; QALY, quality-adjusted life year.
This study evaluated the cost-effectiveness of SZC vs standard treatment for chronic HK in patients with CKD and patients with HF, showing that SZC is cost-effective from the perspective of the Spanish SNS. To our knowledge, this is the first European study to analyse the cost-effectiveness of SZC for patients with HF, and the first in Spain to analyse the cost-effectiveness of SZC, meaning that our results may be very useful for decision-making. Despite the paucity of economic evaluations of the use of SZC in the literature, the results of our study are in line with those obtained in a previous evaluation of a cohort of patients in Norway and Sweden, although that study only looked at treatment in patients with CKD.47
Our analysis estimated an ICER of 5,387/LYG and 9,666/LYG, and an ICUR of 7,605/QALY and 15,078/QALY in patients with CKD and HF, respectively. In both models, although the cost associated with SZC drug treatment was higher, mainly due to the higher acquisition cost of SZC (difference in SZC drug cost vs standard treatment: CKD 11,608; HF 9,737), the cost associated with HK episodes was less, due to the lower rate of these episodes in patients treated with SZC (difference in cost of HK episode treatment, SZC vs standard treatment: CKD −17,688; HF −7,523), as HK episodes are associated with a higher use of resources.3
The results also showed an increase in both LYG and QALY with SZC treatment; 0.671 and 1.526 LYG and 0.476 and 0.978 QALY in patients with CKD and HF, respectively. This improved effectiveness in both cases was mainly due to the fact that SZC treatment improves the prognosis of both diseases, meaning that patients spend longer in the less severe health status levels. We must also take into account that this leads to lower mortality rates in patients receiving SZC, also aided by the lower frequency of HK episodes, which increase mortality risk.3 This means that patients treated with SZC use resources for a longer period of time. This would explain why, in patients on SZC treatment, no savings are observed in the costs associated with disease events, MACE and hospitalisations, or in the costs generated from changes in RAASi therapy. A retrospective real-life study on resource use associated with SZC treatment was recently published in the United States, in which data from more than 1,100 HK patients were analysed, reporting a lower rate of hospitalisations in patients treated with SZC long-term (>90 days) vs those treated short-term (≤90 days).48 This may be associated with a lower cost, but the authors do not provide such data.48 The results indicate that long-term SZC therapy could lead to a reduction in resource use and also in the associated total cost vs standard therapy. Furthermore, according to the authors, long-term therapy could help to optimise RAASi therapy, as suggested by international guidelines.49,50 Similarly, a study conducted in Spain in patients with chronic HK and CKD, HF or diabetes found that most patients (70.4%) were on RAASi therapy, which was frequently discontinued (the higher the severity of HK, the higher the discontinuation rate). Discontinuation of RAASi therapy is associated with an increased risk of AE and disease progression.51–53 In that study, patients with severe HK had a higher risk of hospitalisation and incurred higher annual costs vs patients with mild HK (12,705 vs 4,468).11
The use of SZC in patients with HK could improve plasma potassium control, reducing the high associated costs, as well as ensuring the maintenance of RAASi therapy, making it a therapeutic alternative to resins, whose long-term effectiveness and safety have not been demonstrated. The results of the study also show an improvement in the quality of life of patients treated with SZC.
The main limitations of our study are related to the design of the analysis which, being a modelling exercise, is based on available data and requires that some assumptions have to be made. As usual in our setting, with the exception of costs, most of the data considered came from international sources due to the lack of Spanish studies. Specifically, baseline population characteristics, including patient age and percentage of females, were obtained from the HARMONIZE trial13 (conducted in patients with CKD, HF and diabetes), with both of these parameters lower than those reported in the real-life literature in Spain.11 However, the sensitivity analysis performed on both models shows that, with the variations made, the results remain stable and the conclusions reached are robust. Moreover, since no trials have been conducted directly comparing the efficacy of SZC and the standard treatment considered (calcium polystyrene sulfonate and lifestyle change), the results of the placebo arm of the HARMONIZE trial13 during the maintenance phase (after treatment of the HK episode) were assumed. Also, the HARMONIZE trial13 did not consider lifestyle change (such as a low K+ diet) in the placebo group. In any case, we believe that the assumption reflects actual clinical practice, given the limited evidence supporting the efficacy of such diets and lack of patient adherence. In reference to HK episodes, despite knowing that they are associated with AE,2,54 they have not been considered to have a disutility value. However, this assumption could be considered conservative due to the higher number of HK episodes in patients receiving standard treatment. In addition, we considered an HK event to have occurred when K+ levels were ≥5.5 mEq/l, in line with the indication with funding in Spain.55 Lastly, we did not consider indirect costs, as the analysis adopted the Spanish SNS perspective. However, given the average age of the population considered (>60 years), potential productivity losses could be considered minor. Spanish studies have highlighted that the non-health-related costs associated with advanced stages of HF can be as high or higher than the actual healthcare costs.45 A study in the Nordic countries also found that progression of CKD increases the need for informal care for patients.56 Better monitoring of the progress of patients with HF or CKD can maintain or improve their disease course, which may also have an impact on their non-healthcare needs. Future studies should address the comparison of health outcomes and costs, taking into account the dual perspective of health and social funders.
Despite the limitations, we believe that the analysis can be applied to the Spanish population and clinical practice in Spain, as the assumptions were conservative, a group of experts has validated the data used and the results of the analysis remained stable in all sensitivity analyses performed, representing a cost-effective option vs standard treatment. Additionally, as a strength, it should be noted that it was not necessary to extrapolate efficacy data from the clinical trial, since data from the open-label, long-term phase III trial of SZC were available,16 in which daily use of SZC was associated with maintenance of normal blood-potassium levels, with no substantial change in RAASi therapy in 12 months. Another strength of our study lies in the design of the models; being patient-level simulation models and not the simulation of a cohort as a whole results in a simulation that is closer to reality.
ConclusionsBy preventing episodes of HK in patients with CKD or HF, treatment with SZC reduces the use of healthcare resources and associated costs, while improving patients’ quality of life. The analysis shows that SZC is a cost-effective alternative for the treatment of HK in patients with CKD or HF, taking into account the reference efficiency values commonly used in Spain.
FundingThis study was funded by AstraZeneca.
Author contributionsAll authors of the manuscript (RAA, MCL, JB, JO, MSM, SG, ALM, BLC, NVV, SA and MC) contributed to the conception and design of the project and to the interpretation of the data; they participated in the critical review of the intellectual content of the manuscript and approved the final version presented.
Conflicts of interestRAA declares having received remuneration for this manuscript from AstraZeneca; consulting fees from AstraZeneca, Bayer and Boehringer Ingelheim; payments or fees for lectures, presentations, conferences, manuscript writing or educational events from CSL Vifor, Boehringer Ingelheim and AstraZeneca; grants to attend meetings and/or travel from CSL Vifor, Fresenius and AstraZeneca; and declares holding a paid or unpaid directorship or trustee position in the Sociedad Madrileña de Nefrología (SOMANE) [Madrid Society of Nephrology].
MCL declares having received remuneration for this manuscript from AstraZeneca; payments or fees for talks, presentations, conferences, manuscript writing or educational events from AstraZeneca, CSL Vifor and Boehringer Ingelheim; and grants to attend meetings and/or travel from Pfizer.
JB declares having received consultancy and/or speaking fees and/or travel costs for meetings from Amgen, Abbvie, Sanofi, CSL Vifor, AstraZeneca, Rubio and GSK; and financial support from AstraZeneca for educational activities.
JO declares having received remuneration for this manuscript from AstraZeneca; payments or fees for talks, presentations, conferences, manuscript writing or educational events; and support to attend meetings and/or travel from AstraZeneca.
MSM, SG, ALM and BLC are employees of AstraZeneca. NVV and SA work for an independent research body and have received fees for their contribution to the development of this study.
MC declares having received payments or fees for lectures, presentations, conferences, manuscript writing or educational events from AstraZeneca, Vifor Pharma, Novartis, Boehringher Ingelheim, Bayer and Novonordisk.