Fabry disease or also called Anderson-Fabry disease (FD) is a rare disease caused by pathogenic variants in the GLA gene, located on the X chromosome. This gene is involved in the metabolism of glycosphingolipids and its pathogenic variants cause a deficit or absence of α-galactosidase A causing the deposition of globotriaosylceramide throughout the body. Females have a variable phenotypic expression and a better prognosis than males. This is due to the X chromosome inactivation phenomenon. We present a clinical case of Fabry disease in a female with predominantly renal involvement and demonstrate how the X chromosome inactivation phenomenon is tissue dependent, showing preferential inactivation of the mutated allele at the renal level.
La enfermedad de Fabry o también llamada de Anderson-Fabry (EF) es una enfermedad rara, causada por variantes patogénicas en el gen GLA, localizado en el cromosoma X. Este gen interviene en el metabolismo de los glucoesfingolípidos y variantes patogénicas en el mismo causan déficit o ausencia de la α-galactosidasa A ocasionando el depósito de globotriaosilceramida en todo el organismo. Las mujeres presentan una expresión fenotípica variable y de mejor pronóstico que los varones. Esto es debido al fenómeno de inactivación del cromosoma X. Presentamos un caso clínico de enfermedad de Fabry en una mujer con afectación predominantemente renal y demostramos cómo el fenómeno de la inactivación del cromosoma X es tejido dependiente, mostrando una inactivación preferencial del alelo mutado a nivel renal.
Fabry disease (FD) is an X-linked lysosomal storage disease caused by mutations in the GLA gene, which lead to deficient or absent activity of the enzyme α-galactosidase A (α-Gal A). This leads to an accumulation of glycosphingolipids such as globotriaosylceramide (Gb3) and its deacylated form, globotriaosylsphingosine (lyso-Gb3), in plasma, urine and in the lysosomes and cytosol of various cell types (vascular endothelial cells, podocytes, cardiomyocytes, arterial smooth muscle cells, etc). The GLA gene is located at Xq22.1 and there are currently more than 900 different pathogenic variants described in this gene, most of them missense.1–3 The incidence of classic FD is between 1:40,000 and 1:117,000 live-born males and 1:3,200 if late-onset phenotypes are included.1–4
Accumulation of Gb3 and lyso-Gb3 is associated to smooth muscle cell proliferation, vascular remodelling, oxidative stress, activation of innate immunity and release of pro-inflammatory cytokines.1,5 The phenotype of the disease depends on the residual enzyme activity. The absence or <1% level of α-Gal A results in “classic Fabry”, with paediatric onset and multi-organ involvement, while residual enzyme activity results in late-onset forms.
The clinical manifestations of classic Fabry affect the nervous system in the form of chronic neuropathic pain and attacks of severe pain (“Fabry crises”) and paraesthesia in early life, and in adults, it can present in the form of transient ischaemic attacks or ischaemic strokes. Angiokeratomas, hypohidrosis and heat intolerance, and facial dysmorphism are common. At the gastrointestinal level patients may have abdominal pain, diarrhoea or constipation. Corneal verticillata1–6 is almost a constant in the classic forms, and, like most of the symptoms except for cardiac symptoms, is much less common in the late-onset forms. Renal and cardiac involvement (ventricular hypertrophy, endomyocardial fibrosis, arrhythmias) occur at older ages.
Renal involvement can range from microalbuminuria and proteinuria to the development of progressive renal failure and the need for renal replacement therapy. The finding of podocytes in the urine is the earliest renal manifestation, preceding the development of proteinuria.1,3,4,6
The variability in levels of residual activity of the enzyme α-Gal A correlates with the phenotype which can range from asymptomatic to severe in the classic forms.1,5,6 Late-onset forms show preferentially a cardiac phenotype.
Diagnosis of this disease is often complex and late, with a mean age at diagnosis of 23 years in males and 32 years in females.2 FD should be suspected from clinical evidence and/or family history. In males, the determination of α-Gal A activity is sufficient to make a diagnosis, while in females, genetic testing is necessary, as they may have α-Gal A activity within the normal range.3,4,6 Evidence of lysosomal Gb3 accumulation in renal or cardiac biopsies, although invasive, may be necessary when clinical manifestations or interpretation of the variant in the GLA gene are inconclusive.4 Biomarkers such as urinary Gb3 and blood Gb3 and lyso-Gb3 may be useful for diagnosis and, with less certainty, for monitoring treatment.5
For many years, females with FD were considered to be only carriers of the disease, but it has been shown that they can present with a wide clinical spectrum, ranging from asymptomatic to severe cases similar to those in males, despite normal enzyme activity.1 The most plausible explanation for phenotypic variability in females is X-chromosome inactivation (XCI) or lyonisation, a process by which females make equal the gene dosage of XY males by inactivating one of their X chromosomes in each cell.1–4,6,7 Other factors such as modifier genes or epigenetic or environmental factors may also contribute to the variable expression of the disease in females.
We present a clinical case of FD with predominantly renal involvement and minimal clinical expression in other areas. We demonstrate the clinical correlation with XCI in the kidneys.
Case reportThis was a 35-year-old woman diagnosed at 17 years of age after Fabry disease being found in parent (father) with pathogenic variant in exon 7 of the GLA gene (NM_000169.3): c.1102delinsTTATAC, p. (Ala368Leufs*25). The patient had no relevant previous medical history, however she complained of a long history of symptoms of occasional pain in her lower limbs and dyshidrosis in both hands. She had no angiokeratomas or other skin changes. At the time of the study, she had proteinuria of 500 mg/24 h, without microhaematuria, normal renal function, and cardiac function study with echocardiography showed no abnormalities. Her plasma levels of α-Gal were 70%. She was assessed by ophthalmology with a finding of cornea verticillata. With these findings, it was decided to admit the patient for renal biopsy, given the significant degree of proteinuria in a young woman with FD, in order to rule out other causes of proteinuric nephropathy.
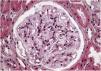
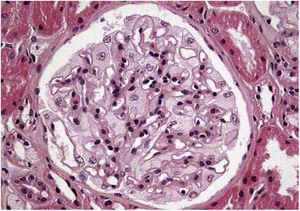
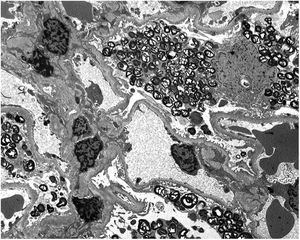
Renal biopsy results show (Fig. 1) increased focal and segmental mesangial matrix with increased cellularity. Both endothelial and epithelial cells show large, multivacuolar cytoplasm. Tubular foam cells are also seen. No interstitial or vascular involvement was identified. Electron microscopy (Fig. 2) shows a massive number of myelin-like inclusions predominantly located within podocytes, with a minimal proportion of such inclusions in endothelial, mesangial and tubular cells. The results lead us to the diagnosis of FD and it was decided to start enzyme replacement therapy (ERT) with agalsidase beta and angiotensin-converting enzyme inhibitors (ACE inhibitors).
In view of the patient's marked renal involvement with little involvement of other organs (except cornea verticillata), a study was carried out of the XCI level in different tissues: peripheral blood, hair, buccal mucosa and kidney. For this purpose, the XCI pattern was analysed by transcriptional analysis of the CAG polymorphism of the androgen receptor gene, using previously reported methodology.8 A similar level of expression of both X-chromosome alleles (mutated and wild-type) was found in all tissues analysed, except for the kidney, which showed a significantly higher expression of the mutated allele (Fig. 3).
Determination of the inactivation status of the X chromosome (XCI) in different tissues of the patient. The first panel shows the amplification of the AR gene in the genomic DNA carried out to correct the preferential amplification of the smallest allele (allele 1). The rest of the panels show the expression of both AR gene alleles in blood, capillary roots, buccal mucosa and kidney. The table on the right shows the quantification of the relative expression levels of both AR alleles, showing random inactivation of the X chromosome in all samples analysed except kidney (>% of the mutated allele).
The patient has been on ERT for 18 years. During follow-up, she remained stable from a renal point of view, with glomerular filtration rates above 90 ml/min/1.73 m2 (CKD-EPI) and proteinuria ranging from 300 to 800 mg/24 h. She continues to be monitored by cardiology without having developed any abnormalities in conduction or cardiac function. The latest cardiac imaging tests show an abnormally reduced native T1 value in the mid-inferior-lateral segment of the left ventricle, compatible with myocardial lysosomal fatty deposits attributable to the FD.
DiscussionIn about 70% of cases, clinical manifestations of FD in females have a high degree of phenotypic variability; this is mostly due to XCI.1 X-linked inherited diseases, such as Fabry disease, generally manifest more severely in males than in females. This is because males are hemizygous for the genes on the X chromosome (they only have one copy), while females, having two X chromosomes, maintain enzymatic activity. In females, the phenomenon of inactivation of the X chromosome occurs during the first week of embryonic development. The inactive X can be the paternal or maternal one and it is inactivated randomly, but permanently. Normally, random assignment of XCI causes 50% of the X chromosomes of maternal origin and 50% of those of paternal origin to be inactivated, resulting in a mosaic of cells, which express one of the two X chromosomes. However, a small percentage of females will have a skewed XCI with >75% expression of one of the two Xs; and in a much smaller percentage (5%) an extremely skewed XCI > 90%.1,4,7,9–12 The percentages of skewed XCI vary depending on age and tissue type, being estimated at around 5%–14% in newborns, 14% in women ≤25 years of age, 16%–37% in women over 60 years of age and 49% in centenarians.1,9 The tissues descending from each of these embryonic cells maintain the same XCI pattern.1,13
In FD, 50% expression of the functional enzyme would theoretically be sufficient to not present severe symptoms of the disease, as there is metabolic cooperation between the cells that express the wild or mutated X chromosome, with enzyme delivery to the mutated cells.1,9,14 Alternatively, cells deficient in the enzyme may divide less efficiently, eventually being replaced by non-deficient cells, which is called cellular selection.11,14
In the case of FD, α-Gal A is taken up by mannose-6-phosphate receptors on the plasma membrane to enter deficient cells by endocytosis. However, some studies suggest that metabolic cooperation is poor in this disease (insufficient secretion of α-Gal A, glycosylation defect of the enzyme, poor absorption) and that the majority of patients with FD and random XCI will develop increasing symptoms with age.9,10,14
Several studies, such as that of Řeboun et al., performed with 35 females patients with FD (26 with random XCI and nine with skewed XCI), or Echevarria et al., with 56 females patients (30% with skewed XCI), found a correlation between XCI pattern and α-Gal A activity.9,12 The recent study by Řeboun et al. detected no relationship between the type of variant and α-Gal A activity in females (finding patients with severe truncating or missense variants and high enzyme activity). They also observed the effect of ageing on the XCI pattern, with changes in two out of eight patients at 6–10 years.12 Data on the impact of XCI on FD are not consistent across studies and evidence is still lacking.
The concordance of XCI in different tissues has been studied in the general population with variable results and very few studies to date in FD. Echevarria et al. studied the pattern of XCI in samples of peripheral blood, buccal mucosa, skin and urinary epithelium in urine sample. They found a skewed XCI pattern in 30% of the cases and a statistically significant correlation between the XCI pattern in blood and that of the rest of the samples.9 However, in this study, the skewed XCI detected in the kidney was not found in the rest of the tissues, correlating with the mainly renal involvement in our patient. Similarly, in the Řeboun et al. cohort, discordances in XCI patterns were found in the different samples of six out of 15 patients (40%).12 This is compatible with the discrepancy in XCI between the different pluripotent stem cells, which leads to the involvement of certain organs, which can potentially be severe, with less involvement of others.
The XCI process is still far from being fully understood, but it seems clear that it is a dynamic and complex phenomenon. This complexity includes a proportion of genes which escape XCI, tissue-specific and cell-to-cell differences in the XCI process, and the intricate gene- and gene-region-specific role of DNA methylation exerted on both the active and in the inactive X chromosomes. An important regulatory role has been determined at the transcriptional level of X mediated by the X-inactive specific transcript (XIST) gene,10,15,16 as well as at the level of protein translation or by epigenetic mechanisms (DNA methylation, histone modification, etc).15–17 Inactivation begins at the X chromosome inactivation centre (XIC), where untranslated XIST RNA accumulates and coats the future inactivated X chromosome, followed by chromosome-wide epigenetic changes. In somatic cells, the inactivated X chromosome is visible as the Barr body.18
Although some bias in X inactivation could be attributed to variants in the XIST gene (involved in familial cases of skewed XCI), such variants occur only rarely,14 with cellular selection and random bias (given the small number of cells present at the time of implantation) being considered as the most common causes of skewed XCI.1,11,14 There are still many unresolved questions, in particular regarding the timing of initiation and reversal of XCI, the sequence of molecular events and the regulatory network of XIST and the rest of mechanisms responsible.15
Most studies investigating the role of DNA methylation in FD analyse the (human androgen receptor (HUMARA) gene, as it is quick and inexpensive.19 However, it is important to note that study of the HUMARA gene cannot distinguish between the two chromosomes and cannot therefore predict disease severity in a female with highly skewed XCI. Furthermore, classic approaches to measuring skewed XCI may not be sufficient to explain disease manifestations in female patients. Allele-specific DNA methylation in the promoter region of the GLA gene may also influence the expression levels of the mutated allele, with implications for the severity of FD. Therefore, approaches that distinguish between the mutated and non-mutated allele by analysing DNA methylation at the GLA promoter may be much more informative. The study of XCI is not something generally performed in clinical practice, and there is not sufficient evidence to recommend it. Despite this, it has been included as a criterion for starting specific treatment for FD.4
The case we present here demonstrates the lack of correlation of XCI in peripheral blood with what is occurring in other organs. Although we are not proposing that it should become routine clinical practice, it is interesting from a pathophysiological point of view.
FundingNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.