Primary tubular disorders are rare. Most of them have an autosomal recessive inheritance pattern. Thanks to modern molecular biology techniques we have been able to identify the intimate genetic cause of these diseases, and discovered that these disorders are more common in the areas where, in the past, there was a greater or lesser degree of consanguinity. Population displacements since the dawn of humanity are the cause of the spread of some diseases of genetic origin. Knowledge of these allows researchers to follow the journeys that certain chromosomal mutations have taken from the place of origin of the “founding ancestor” to other locations, often in very distant parts of the planet.
In this article, based on our own and others’ results, we review some of the available findings on the molecular basis of certain tubular disorders and two kidney stone-causing renal disorders in relation to inbreeding and population displacements.
ConsanguinityThe consequences of inbreeding are an essential component for science. Since Mendel's pea plants, inbreeding has been contributory in numerous genetic discoveries. In the area of renal medicine, nephrologists are familiar with congenital nephrotic syndrome, Finnish type, caused by mutations in the gene that codes for nephrin.1 It was described in the 1970s by nephrologists from Finland,2 which has one of the highest rates of consanguinity in the world.3 It is important to know whether or not the impact of inbreeding on human health has been exaggerated. It is true that in a marriage between first cousins the risk of a recessive illness is doubled (around 5%) compared to non-blood parents (2–3%).3 In the 1960s, studies in island populations in Japan showed no clear relationship between inbreeding and overall health.3 Years later, it was shown that mental retardation and lower scores on tests that measure the IQ4 were more likely in consanguineous marriages. As we discuss below, on the Canary Island of La Gomera, a higher frequency of two causes of kidney stones has been reported which must be related to the high rate of inbreeding that existed on the island until at least the 20th century. The rate of marriages between first cousins may have been as high as 25.8%.5
La Gomera, “the island of stones”The findings made in recent years in the present inhabitants of the island of La Gomera have become a paradigm of the medical difficulties that can be related to consanguinity, although not all of them are probably negative. They have found, on the one hand, a higher frequency of a serious cause of lithiasis and chronic kidney disease, hyperoxaluria type 1 and, on the other, a metabolic abnormality, idiopathic hypercalciuria, which can cause kidney stones and loss of bone mass in some cases.
La Gomera is one of the western Canary Islands and one of the smallest. It currently has a population of just over 20,000. The island is formed by steep volcanic mountains with deep ravines radiating from the centre, which makes communication between the different areas difficult. This special geography led to the development of a special form of sound language called “silbo gomero” [Gomeran whistling], of such importance that it was awarded World Intangible Cultural Heritage status in 2009.
From the 1990s, a higher rate of primary hyperoxaluria type 1 had been observed in the adult population of the island of Tenerife.6 The same was reported later in the island's paediatric population.7 In 2003, members of the Canary Islands Hospital Universitario Nephrology Department identified the c.853T>C (p.I244T) mutation in most of the AGXT gene alleles of patients with primary hyperoxaluria type 1, many of whom had ancestors on the island of La Gomera. Although the p.I244T mutation did not affect the activity of AGXT or its subcellular location, when the c.32C>T (p.P11L) polymorphism was present in the same protein molecule, it resulted in the loss of its enzymatic activity in soluble cell extracts.8 The results were compatible with a founder effect, i.e. originating from a common ancestor. The inheritance pattern of this disease is autosomal recessive.
The case of idiopathic hypercalciuria (IH) is different. It is not considered a disease but a “metabolic abnormality”, or rather, a constitutive characteristic with which patients would inherit the disposition towards having a higher number of vitamin D receptors (VDR) in their cells than people with normal urine calcium excretion. The pathophysiology of IH has been summarised in a recent article.9
In the 1990s, we found that the paediatric population of the island of La Gomera had a very high rate of IH; in fact, one of the highest in the world (16% vs. 3.8% in the control group). The prevalence varied in different populations on the island, but was higher in the most isolated and difficult to access, which could be related to a higher rate of inbreeding than in the more accessible populations.10 Our hypothesis is that the children of La Gomera with IH are descendants of the most immunologically capable subjects,11 which would be those with the highest density of VDR, i.e. a form of “natural selection”. Therefore, insularity and the associated high rate of inbreeding would not be the cause but rather the way to perpetuate these immune conditions favouring the subjects that survived. More details are cited in the aforementioned article.9
Characteristics of patients affected by certain tubular disorders diagnosed on the island of Tenerife (cystinosis, Bartter syndrome type IV, familial hypomagnesaemia with hypercalciuria and nephrocalcinosis)The majority of children with tubular disorders diagnosed in the hospitals on Tenerife come from the island of La Palma and from a specific area in the north west (NW) of the island, which suggests a high degree of consanguinity in those areas.
Until 2009, no cases of cystinosis had been diagnosed in Tenerife. The only patient studied came from the population of Icod de los Vinos, located in the aforementioned geographical area on La Palma.12
In 1995, Landau et al. described five children belonging to an inbred extended Bedouin family who were affected by Bartter syndrome and sensorineural hearing loss. This association was called Bartter syndrome type IV.13 The BSND gene encodes a protein called “barttin” which is co-localised to the chloro-Ka (ClC-Ka) and chloro-Kb (ClC-Kb) channels of the basolateral membranes of the thick ascending limb of the loop of Henle and in stria vascularis cells of the inner ear.14
In 2006 in our hospital, we studied two families, three members of one of which and two members of the other, respectively, were diagnosed with Bartter syndrome type IV. Both families came from the same geographical area of the NW of the island (Icod de los Vinos). In one of the two families, the paternal grandmother of the mother of two patients and the paternal grandmother of the mother of a third patient were sisters. The members of both families denied being related to each other. Sequence analysis of the BSND gene showed that all affected members were homozygous for a C to T transition in exon 1, while their parents were heterozygous. This alteration results in a change in direction mutation, c.139G>A (p.G47R), which had previously been shown to eliminate its favouring effect on the barttin subunit of the ClC-Kb channel.15
Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC) is the only known tubular disorder in which abnormal proteins function in the paracellular space. In 1999, Simon et al. described the existence of a protein, paracellin-1, which is necessary for paracellular tubular reabsorption of magnesium. This protein is located in tight junction areas, intercellular structures which allow intimate contact between adjacent epithelial cells. These authors established that mutations in the PCLN-1 gene which encodes paracellin-1 were the cause of FHHNC.16 The new protein was later renamed claudin-16 (CLDN16 gene). It was subsequently found that there were patients with FHHNC who did not have CLDN16 gene mutations. At the same time, this tubular disorder was found to be very common in Spain. In fact, its definitive name comes from an article published by Manuel Praga et al. in 1995.17 Cesar Loris, a paediatric nephrologist at the Hospital Infantil Universitario Miguel Servet in Zaragoza, confirmed with his colleagues that in the Spanish patients, the rate of eye disorders was unusually high (81% vs. 24% in patients from other countries).18 The fact that, except for a very few, the Spanish patients with FHHNC did not have mutations in that gene was striking.19 That issue was resolved in 2006, however, as other patients with FHHNC were identified who were carriers of mutations in the CLDN19 gene, another member of the claudin multigene family. These children suffered from hypomagnesaemia, chronic kidney disease (in many cases early) and serious eye abnormalities.20 Two years later, Hou et al. demonstrated that both claudins act synergistically through a heteromeric interaction which establishes the electrophysiological environment necessary in the tight junction areas to obtain a selective mechanism of cation reabsorption in the thick ascending limb of the loop of Henle.21 As will be seen later, the majority of Spanish patients with FHHNC are carriers of the same mutation in the CLDN19 gene. However, we only know of the existence of three Spanish patients with a mutation in the CLDN16 gene. One of them comes from another population in the NW of Tenerife (San José de los Llanos).22
Distal renal tubular acidosis, King Carlos II and tropical distal renal tubular acidosisIt has been written about Carlos II, the last Spanish king of the Spanish Habsburg dynasty (1516–1700), that he may have suffered from diseases as diverse as panhypopituitarism and progeria, Klinefelter syndrome, acromegaly and fragile X syndrome. What can be said with certainty is that he had seizures and contracted malaria. He had little musculature and, until he was six years old, he could not walk and could barely stand. He had a big head, which has been attributed to hydrocephalus. He suffered from epileptic seizures, which were exacerbated towards the end of his life. On his activity in his last years it was written that: “His weakness is so great that he cannot remain more than 1 or 2h out of bed. When he gets in or out of the carriage he always needs help”.23 In 2009, Gonzalo Álvarez et al. from the Universidad de Santiago de Compostela awakened the interest of the scientific community with their conclusions about the diseases this unfortunate monarch may have suffered. They calculated that the inbreeding coefficient (F) of the king was 0.254, in other words, a level of inbreeding greater than what would happen to the son of two siblings (F=0.25), meaning that more than a quarter of his DNA was identical in both alleles.24 Of course, this was due to the disastrous policy of consanguineous marriages typical of the reigning dynasty in Spain during the 16th and 17th centuries. Álvarez and his colleagues reported that Carlos II could have simultaneously been carrier of two genetic disorders determined by recessive alleles, namely a combined pituitary hormone deficiency and distal renal tubular acidosis (dRTA). According to these authors, in Carlos II, “His muscular weakness at an early age, the rickets, haematuria and his large head in relation to the size of his body” could be attributed to this genetic disorder, dRTA.24 In the royal post-mortem, it was observed that: “The cadaver did not have even a drop of blood, the heart appears the size of a peppercorn; the lungs corroded; the intestines rotten and gangrenous; in the kidney three large stones, one single testicle, black as coal, and the head full of water”. Of course, it is very difficult to know with certainty what his actual ailments were, but it does not seem likely that he had a dRTA. It is known that the hypokalaemia typical of this disease can cause tetraparesis, but chronic muscle hypotonia can have many other causes, such as Becker-type muscular dystrophy, for example. It is true that the king was of short stature,23 but in his medical history, a lot of which is preserved, there is no mention of any symptoms characteristic of dRTA which would have gone unnoticed by his contemporaries, especially the European ambassadors who reported the royal ailments in full to their respective countries. We refer to polyuria and polydipsia. It is true that he had three stones in his bladder when he died (one, in another version) but, also, the post-mortem on his father Felipe IV revealed the same.23 Therefore, both are more likely to have had idiopathic hypercalciuria, associated or not with hypocitraturia which, as we have mentioned before, is also related to consanguinity. It could be argued that both father and son may have suffered from the autosomal dominant form of dRTA, but it is very unlikely because Felipe IV was of a normal height,23 which is not compatible with chronic metabolic acidosis.
Turning to another matter, the dRTA caused by mutations in the SLC4A1 gene that encodes the erythrocyte and kidney isoforms of anion exchanger 1 (AE1 or band 3) has a high prevalence in some tropical countries, particularly Thailand, Malaysia, the Philippines and Papua New Guinea. In this case, the disease is almost always recessive and can be the result of homozygous or compound heterozygous mutations in the SLC4A1 gene. These mutations can cause morphological changes in red blood cells, often with excessive haemolysis. In contrast, the “classic” dRTA caused by mutations in the SLC4A1 gene is much rarer, almost always has an autosomal dominant inheritance pattern25 and is caused by mutations quite different from those found in the tropics.
Oliver M. Wrong (1925–2012) was an eminent English nephrologist who dedicated much of his life to the study of acid-base balance and associated disorders.26–28 In the last years of his life he devoted himself to trying to explain the reason for the high rate of dRTA in South-east Asia. The result was a publication suggesting the hypothesis that changes in red blood cells caused by these mutations could protect against malaria.29 Indeed, ever since the mid-1970s, the relationship between human erythrocyte variants and susceptibility to malaria infection has been the subject of intensive clinical and epidemiological study.30 In 1981, it was demonstrated that the ovalocytic erythrocytes of Melanesians living in Papua New Guinea were resistant to infection by malaria parasites (Plasmodium falciparum). Analysis of their behaviour on thermal deformation showed that ovalocytes were more thermostable than normocytes, which suggested a significant difference in the structure of the cytoskeleton.31
The Roma people and Gitelman syndromeMolecular genetics studies have clearly distinguished Bartter syndrome from a disease with similar characteristics described in 1966 by Gitelman, Graham and Welt.32 These authors published the clinical data of three adult patients, two of them siblings, affected by hypokalaemia, hypomagnesaemia and metabolic alkalosis. For many years, patients with these characteristics were misdiagnosed as adult Bartter syndrome. The presence of hyperreninaemia and hyperaldosteronism contributed to the confusion with classic Bartter syndrome. In 1996, it was established that Gitelman syndrome is caused by an alteration in the transport of NaCl in the distal convoluted tubule due to the existence of mutations in the SLC12A3 gene which encodes the thiazide-sensitive Na+-Cl−co-transporter (NCC), which is located on the lumen side of the cells of the early distal convoluted tubule.33
The Roma people are an itinerant community with particular genetics. It is believed they come from India and that around 500 AD they settled in the Balkans and dispersed throughout Bulgaria and other European countries. Although it is difficult to estimate the degree of inbreeding in Roma communities, more than nine mutations have been discovered that appear to be specific to their members.3 This is what happens with Gitelman syndrome. The mutation c.1180+1G>T, which consists of the substitution of guanine with thymine at position 1 of intron 9 of the SLC12A3 gene, is a unique mutation exclusive to the Roma ethnic group. It has been studied by the Paediatric Nephrology Group and the Molecular Biology Laboratory at Hospital Universitario Central de Asturias (Doctors Coto and Santos). It has been suggested that it could be a mutation of ancient origin spread throughout Europe in this ethnic group.34 Recently, this same group has identified a second founder mutation of the SLC12A3 gene, c.1939G>A, in gypsy patients who do not have the variant c.1180+1G>T.35 This new mutation involves the exchange of valine for methionine in residue 647 of the NCC protein.
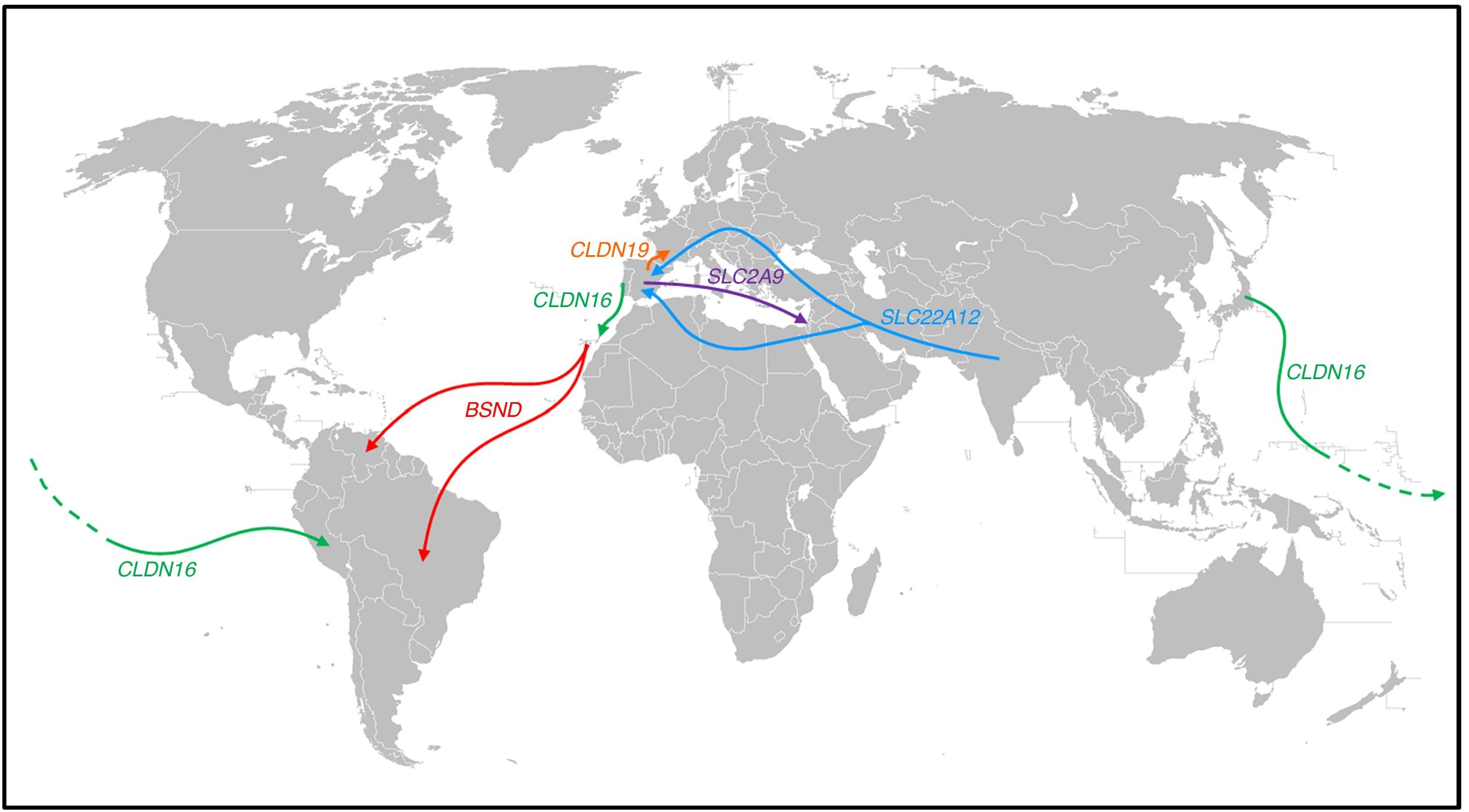
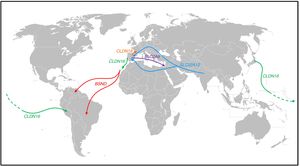
Population movements and some of the findings observed in patients with several tubular disorders (Fig. 1)Bartter syndrome with deafnessThe creation of the RenalTube Group, to improve understanding of primary tubular disorders, has enabled us to study the DNA of patients with tubular disorders who come from outside Spain36 (see portal http://www.renaltube.com/es/). We have been able to demonstrate, for example, that the G47R mutation found in Tenerife patients with Bartter syndrome with deafness15 is the same as in two patients in Venezuela37 and one in Brazil. It is known that there was a high rate of emigration of young Canary Islanders to Venezuela (“the eighth island”) during the first half of the 20th century, especially in the difficult years that followed the Spanish Civil War.
Population displacements and paths followed by subjects carrying certain mutations causing some of the tubular disorders described in the text. The G47R mutation that causes Bartter syndrome has been reported in the NW of the island of Tenerife, as well as in Venezuela and Brazil (red arrows). The p.R149Q mutation identified in the CLDN16 gene which encodes claudin-16 has been reported in both Japan and Peru; in the same gene, the p.G162 V mutation has been reported both in Portugal and on the island of Lanzarote (green arrow). The p.G20D mutation in the CLDN19 gene has been found in the majority of Spanish patients with familial hypomagnesaemia with hypercalciuria and nephrocalcinosis and in French patients of Spanish origin in the south-west of France (orange arrow). The c.1180+1G>T mutation, which causes Gitelman syndrome, has been identified as exclusive to the population of Roma origin (light blue arrows). The p.T125 M mutation found in the SLC2A9 gene has been identified in Spanish patients with renal tubular hypouricaemia and in one of Sephardi origin in Israel (purple arrow). The colours in the figure can only be appreciated in the electronic version of the article.
Another case is that of a patient from the rain forest in Peru who had been diagnosed with FHHNC. The analysis of the CLDN16 gene which, as we said above, encodes claudin-16, detected the p.R149Q mutation in homozygosis. This mutation had only been detected in heterozygosis in a patient from Japan.38 We must remember that at the end of the 19th century there was a wave of emigration of Japanese citizens to Peru to work in agriculture.
From the 15th century there was a lot of Portuguese presence in the Canary Islands. Lanzarote was no exception. During the first half of the 15th century, Portugal struggled with Castile for possession of the island. In fact, the Portuguese Antão Gonçalves was Governor and Captain General of Lanzarote in 1448 and 1449.39 Later, the Portuguese, excluded from the Indies as foreigners but admitted without hindrance in the Canary Islands, replaced the lack of Castilian arms there,40 although sometimes they arrived for more specific reasons, one of which being that they were fleeing the Inquisition.41 Over the following centuries, on the rare occasions the island's agricultural capacity was good thanks to the rains, added to the locals who returned from their “nomadic wanderings” around the other Canary Islands were also people from other parts. For example, we know that in 1640 about 200 Portuguese people arrived in Lanzarote thanks, above all, to the trade between Lanzarote and Madeira. It is therefore not surprising that our RenalTube Group detected the p.(G162 V) mutation in the CLDN16 gene of two siblings from Lanzarote with FHHNC who have already been transplanted, and that this mutation was first identified in a Portuguese patient.42
Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis. Claudin-19With respect to the other gene involved in FHHNC, CLDN19, the original study by Konrad et al. we mentioned earlier included seven Spanish patients belonging to different families in whom they detected the same mutation in homozygosis, p.G20D (c.59G>A), which they called the Spanish/Hispanic mutation.20 These authors suggested that p.G20D is a founder mutation. The study published by Vargas-Poussou et al. described two other unrelated Spanish patients and 13 French patients, belonging to 12 families, with the p.G20D mutation, the majority in homozygosis. The families that came from the south-west of France were of Spanish origin. The data obtained through a microsatellite analysis in these families were consistent with a founder effect.43 Subsequently, our group published the results of the study of a Spanish cohort consisting of 34 patients with FHHNC, 20 girls and 14 boys, members of 30 apparently unrelated families.44 The clinical data for these patients showed a high risk of progression towards end-stage chronic kidney disease (62% with impaired renal function and 20% transplanted), which is consistent with what was found in the French series. Our results are also in line with those of the French group in terms of the high percentage, 88% and 91% respectively, of patients with serious eye abnormalities such as myopia magna, nystagmus and coloboma. In contrast, the nine patients with mutations in the CLDN16 gene studied by the French group, most of whom came from North Africa, had no eye abnormalities. Mutation analysis in our cohort showed that all patients had mutations in the CLDN19 gene. The p.G20D mutation was detected in 93% of unrelated patients and was present in homozygosis in 83% of cases.44
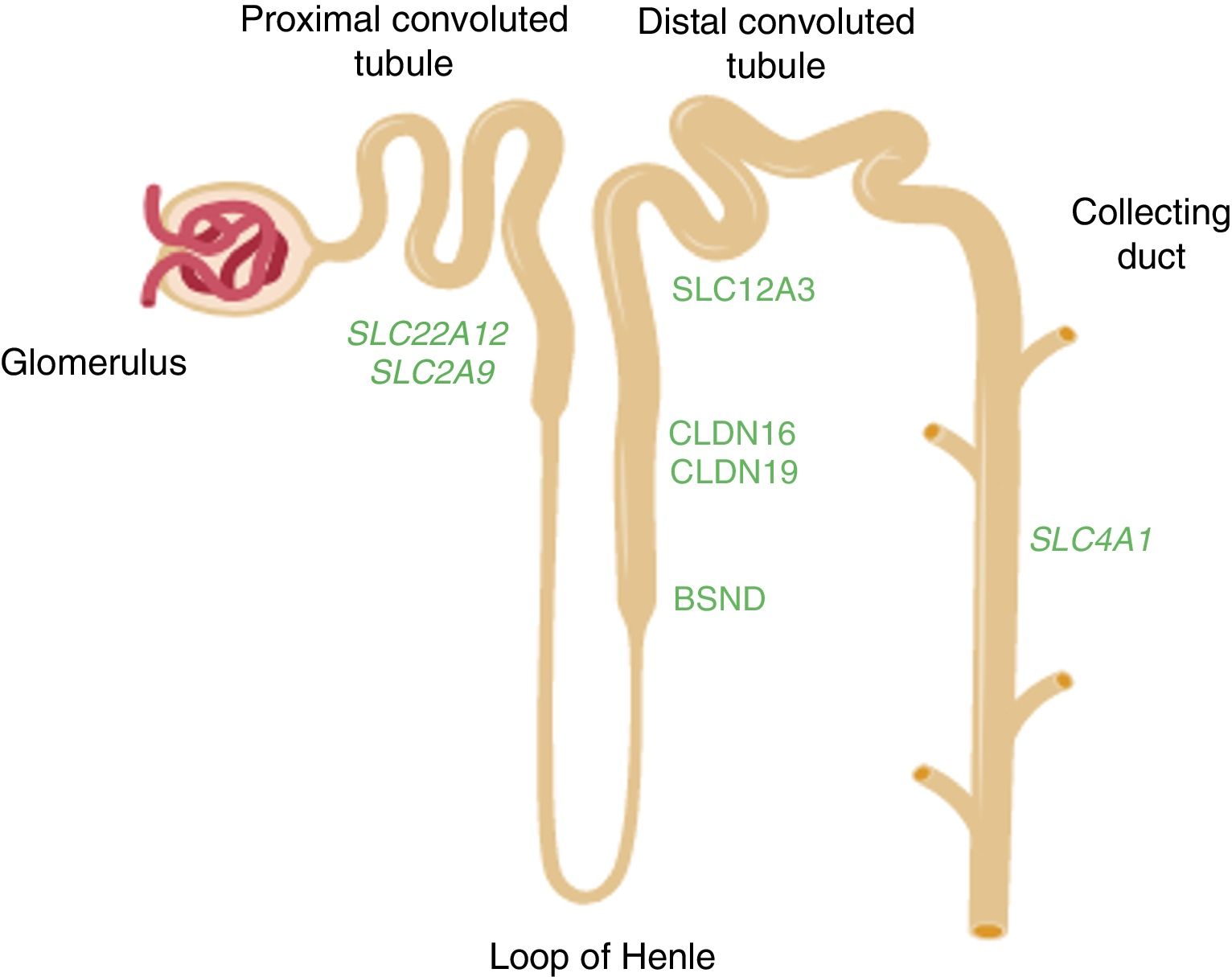
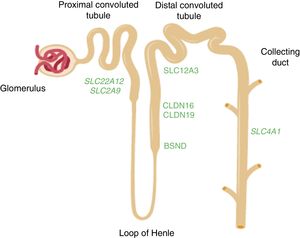
Renal tubular hypouricaemiaIn 1950, Praetorius and Kirk described the case of a patient with a striking hypouricaemia (0.3mg/dl) in which the uric acid clearance was higher than the creatinine clearance. They assumed that this individual's kidney was secreting uric acid. It was the first case to be described of renal tubular hypouricaemia.45 The URAT1 transporter which reabsorbs the filtered urate was identified by Enomoto et al. in 2002.46 It is located in the apical membrane of the proximal tubule cells and is encoded by theSLC22A12 gene. Uric acid is taken out into the peritubular space by basolateral transporters. In 2003, Jutabna et al. identified a new voltage-sensitive organic ion transporter, glucose transporter 9 (GLUT9), which facilitates the exit of urate from the cell47 (Fig. 2). It is encoded by the SLC2A9 gene. In a recent study, our Group studied ten patients with mutations in SLC22A12.48 Two mutations associated with renal hypouricaemia, c.1400C>T (p.T467 M) and c.1245_1253de (p.L415_G417del), are very common in the Roma people in the Iberian Peninsula, suggesting there would be a high incidence of renal hypouricaemia in this population.48,49 In addition, four patients were carriers of the p.T125 M mutation in SLC2A9. This mutation was initially identified in a patient of Sephardi origin living in Israel.50 The expulsion of Jews from Spain was ordered by the Catholic Monarchs in 1492. As some Jews equated Spain with the biblical Sepharad, they were given the name Sephardis. In addition to their religion, they maintained many of their Spanish customs, in particular, retaining their language, which is derived from the Spanish spoken in the 15th century.
Diagram of the nephron showing the segments of the tubule where the genes mentioned in the text are expressed. The SLC22A12 and SLC2A9 genes which encode the uric acid transporters URAT1 and GLUT9, respectively, are expressed in the proximal tubule. The CLDN16 and CLDN19 genes which encode claudin-16 and claudin-19 respectively are expressed in the tight junction areas of the thick ascending limb of the loop of Henle. The BSND gene which encodes barttin is expressed in that same segment of the tubule, but also in the stria vascularis cells in the inner ear. The SLC12A3 gene which encodes the thiazide-sensitive NaCl co-transporter is expressed in the cells of the distal convoluted tubule. The SLC4A1 gene encodes the isoforms of anion exchanger 1 (AE1) in erythrocytes and kidney. In the last case, the protein is located in the renal collecting duct.
Tubular disorders are clinical alterations in which there is a specific tubular dysfunction with little or no involvement of glomerular function.
They are rare hereditary disorders caused by alterations in genes expressed by different parts of the nephron.
Most of these diseases have an autosomal recessive inheritance pattern and tend to be more common in areas which are geographically isolated or have a high degree of consanguinity.
Population displacements are responsible for the spread of some of these diseases. Knowledge of these allows researchers to follow the journeys that certain chromosomal mutations have taken from the place of origin of the “founder ancestor” to other locations, often in very distant parts of the planet.
FundingOur studies were funded by the Instituto de Salud Carlos III [Carlos III Health Institute] – Sub-directorate General for Assessment and Promotion of Research (PI11/00342, PI14/00760, and PI17/00153) and by the European Regional Development Fund “A way to make Europe”. There has been no participation by these institutions in the research itself or the preparation of the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Nieto VM, Claverie-Martín F, Perdomo-Ramírez A, Cárdoba-Lanus E, Ramos-Trujillo E, Mura-Escorche G, et al. Consideraciones acerca de las bases moleculares de algunas tubulopatías en relación con la endogamia y los desplazamientos poblacionales. Nefrologia. 2020;40:126–132.