Alterations in the sense of smell (dysosmia, anosmia, hyposmia) are frequently experienced by patients with chronic kidney disease. However, currently, the aetiology and consequences are poorly understood, with no effective treatments available to address such impairment. In general, the capacity of olfactory perception is affected in patients with chronic kidney disease (even in those who have not undergone dialysis therapy), and whether these alterations improve after dialysis is disputed. Patients in peritoneal dialysis and haemodialysis have the same olfactory perception defects. Kidney transplantation improves olfactory perception, and one important consequence of such impairment is the potential impact on the patient's nutritional status.
Las alteraciones en el sentido del olfato (disosmia, anosmia, hiposmia) son frecuentes en los pacientes con enfermedad renal crónica; sin embargo, hasta el momento actual las causas, consecuencias y tratamiento de estas alteraciones han sido poco abordadas. Los pacientes con enfermedad renal crónica sin tratamiento de diálisis muestran disminución en la percepción olfativa y existe controversia sobre si estas alteraciones se corrigen con la diálisis. El grado de percepción olfativa es similar cuando se compara la población en diálisis peritoneal y en hemodiálisis. El trasplante renal corrige estos déficits olfativos. Una de las probables consecuencias de esta afección es el impacto en el estado nutricional del paciente.
Alterations in the sense of smell such as decreased sensitivity and perception (dysosmia, anosmia, hyposmia) are a common, but generally not well recognised, problem. The prevalence of these alterations increases significantly in older adults above the age of 70. In the United States, according to the National Health and Nutrition Examination Survey (NHANES III), the prevalence of dysosmia in the general population is 12.4%, while 3% have anosmia. However, over the age of 80, 39% have some degree of olfactory dysfunction.1
In addition to age, the perception of smell is affected by different diseases (diabetes, neurodegenerative diseases, respiratory diseases, cancer, etc.), trauma, medication, exposure to herbicides, solvents and radiation, and a person's nutritional status.2
The objective of this review was to analyse the prevalence, pathophysiology and treatment of olfactory disorders in patients with chronic kidney disease (CKD). Very few studies have examined the aetiology and pathophysiology of olfactory alterations in this group of patients, so at present there are no adequate treatments to address this problem.
Olfactory perceptionThe nasal cavity is lined with somatosensory receptors (pain, heat, pressure) for the trigeminal, glossopharyngeal and vagus nerves. However, chemosensory signals are mediated only by the olfactory nerve.
Odour molecules in the air enter the nose through the nostrils where they dissolve in the secretions from the mucosa that lines the nasal cavity. These molecules then bind to olfactory sensory neuron receptors. The number of odour molecules in the environment is greater than the number of receptors we have in the nose. In humans, 3% of all genes code for olfactory receptors, with approximately 450 types of olfactory receptors. Therefore, a molecule can stimulate a combination of receptors and thus create a unique representation in the brain. The brain registers each of these representations as a particular smell.
The primary processing of olfactory signals occurs in the olfactory bulb. The non-myelinated axons of the olfactory sensory neurons ascend through the perforations of the cribriform plate of the ethmoid bone and synapse in the olfactory bulb. About ten million neurons process odours in the olfactory bulb.3 After passing through the olfactory bulb, the olfactory information is transmitted to higher brain centres within the primary olfactory cortex (including the anterior olfactory nucleus, the olfactory tubercle, the piriform cortex and the periamygdaloid cortex) and the limbic system (hypothalamus, hippocampus, amygdala), enabling multiple signals to be processed to form an olfactory perception.4,5
Assessment of olfactory functionMost tests to assess olfactory function are based on the identification and discrimination of a variety of odours. In general, the study of olfactory ability is carried out by performing three tests: (a) evaluation of the odour threshold, in which the minimum concentration necessary for a smell to be perceived by a person is determined; (b) olfactory discrimination, in which a person's ability to differentiate one smell from another is assessed; and (c) identification of the smell, where it is determined whether the person can recognise a smell.
The assessment of olfactory ability is complex as the simple identification of odours depends to a large extent on the verbal skills of the subject. In addition, smell identification tests have a strong cultural connotation since not all smells are recognised in the same way by different peoples (cultural connotation of smells).6,7
Sense of smell in chronic kidney diseaseThe sense of smell is extremely important for communication, for establishing relationships between individuals, for detecting and appreciating food, for the timely detection of toxic substances, etc. The perception of smell decreases with age and is affected by medications, malnutrition and diseases such as diabetes. Studies in patients with CKD have shown that olfactory system disorders are common in this population (Table 1).
Summary of olfactory function studies in patients with chronic kidney disease.
| Author | Subjects (n) | DM2 (%) | Threshold | Discrimination | Identification | Anosmia (%) |
|---|---|---|---|---|---|---|
| Schiffman et al.10 | 27(HD=11, C=16) | ND | Reduced in patients on HD | ND | ||
| Vreman et al.8 | 81(HD=26, CKD=7, C=48) | ND | Not significantly elevated in patients with CKD | ND | ||
| Griep et al.9 | 101(HD=38, PD=16, RT=28, CKD=19) | ND | Deficit in patients with CKD, HD and PDImproved in patients with RT | ND | ||
| Frasnelli et al.11 | 79(HD=49, CKD=15, C=15) | ND | No changes compared to the control population | Decrease | Deficit in patients with CKD and HD | 4.7 |
| Raff et al.12 | 49(HD=31, C=18) | 48 | Control subjects better than HD | ND | ||
| Landis et al.13 | 52(HD=20, PD=8, C=24) | ND | Deficit in patients with HD and PDa | Patients on PD scored better than HD | ND | |
| Koseoglu et al.14 | 107(CKD=30, HD=38, PD=15, C=24) | 0 | PD better than HD better than CKD | Deficit in patients with CKD, HD and PD | Patients on PD scored better than HD. Both groups better than CKD | 8.4 |
| Nigwekar et al.15 | 161(CKD=36, HD=94, PD=6, C=25) | 50 | Deficit in patients with HD and PDNormal in CKD | Decrease in CKD and end-stage renal disease | 10 |
C: healthy control; CKD: chronic kidney disease; HD: haemodialysis; PD: peritoneal dialysis; RT: renal transplant.
In one of the first studies conducted to assess the sense of smell in a population with CKD (haemodialyses [HD]), Vreman et al.8 found that olfactory perception was diminished (although not significantly) in male patients; but this deficit was not related to the amount of zinc in blood and hair (Table 2).
Summary of variables which can affect olfactory function in patients with chronic kidney disease.
| GFR | Clear association between decreased GFR and greater olfactory deficits |
| Kt/V | Apparently no association with olfactory alterations |
| Urea | Conflicting studies:Griep et al.10: association between urea levels and olfactory deficits but other studies have not corroborated this13 |
| Malnutrition | Olfactory deficits associated with malnutrition markers (e.g. SGA, PCR) |
| CRP | Inconsistent findings:Raff et al.12: association between CRP levels and olfactory deficitsKoseoglu et al.14: no association |
| Uraemic toxins | Olfactory deficits not associated with uraemic toxins (P-cresol, etc.)12 |
| Treatment | Studies show conflicting results on whether or not dialysis improves olfactory deficits; in some olfactory tests, PD patients have lower olfactory deficits than HD patients.Landis et al.13: Identification and acetic acid thresholds improved after haemodialysisGriep et al.10: No improvement with the treatment (isoamyl acetate [banana/pear]) |
CRP: C-reactive protein; GFR: glomerular filtration rate; HD: haemodialysis; PCR: protein catabolic rate; PD: peritoneal dialysis; SGA: subjective global assessment.
Griep et al.9 studied 101 patients with CKD (38 on HD, 16 on peritoneal dialysis [PD], 28 renal transplant recipients and 19 without dialysis with glomerular filtration rate ≤30ml/min), and found that the olfactory threshold was lower in patients on PD and HD and those with CKD with glomerular filtration rate ≤30ml/min. The results of the olfactory tests in the transplanted subjects were similar to the control group and there was no difference between subjects on HD vs PD; in HD patients, there were no changes in olfactory perception between the pre- and post-dialysis assessments. Serum urea and phosphorus levels were inversely related to the subjects’ olfactory abilities, which suggests that the olfactory system may be affected by the high concentration of these elements. However, the fact that olfactory perception only improves by transplantation and not by dialysis suggests that regulation of this function is complex.
Frasnelli et al.11 found olfactory deficit in 56% of 49 patients with CKD on HD. In the olfactometry tests performed, 11% of the patients had an increase in the olfactory threshold, 38% reduced discrimination of odours and 48% deficits in odour identification.
Raff et al.12 studied the relationship between alterations in olfactory tests, clinical parameters of malnutrition and levels of uraemic toxins such as monomethylamine, ethylamine, indoxyl sulfate, and P-cresol sulfate in 31 patients with CKD on HD. The study demonstrated the association between olfactory dysfunction, higher levels of C-reactive protein and patient malnutrition. No correlation was found between the degree of olfactory dysfunction and serum levels of albumin, cholesterol or uraemic toxins. The results of this study show the contribution of the process of malnutrition and inflammation in altered olfactory function in patients with CKD.
Landis et al.13 performed olfactory tests before and after each HD session to determine whether or not HD modified olfactory function, and found that olfactory ability assessed by olfactory function tests had improved after the session. They also reported greater deterioration in olfactory function in patients on HD compared to patients on PD.
In non-diabetic patients at different stages of CKD (CKD not on dialysis, HD and PD), Koseoglu et al.14 reported greater deterioration of olfactory function in patients on PD.
Nigwekar et al.15 demonstrated a greater olfactory deficit in the identification and discrimination tests, with normal olfactory threshold in patients with CKD compared to the control group. They also demonstrated the correlation between olfactory deficit and patient nutritional status.
Whether or not there is a progression in the olfactory deficit with to the deterioration of renal function remains uncertain. There is also a lack of conclusive evidence on which is the most effective dialysis modality for improving the deficit in olfactory function. The different studies do however show evidence of the relationship between nutritional status and olfactory function in patients with CKD.
Malnutrition is associated with a decrease in serum levels of vitamins (e.g. vitamin B12) and trace elements which also affects patients’ olfactory function. At the same time, the decrease in olfactory ability predisposes to lower food intake and increased malnutrition.13,15
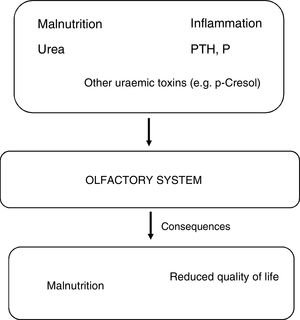
PathophysiologyStudies dedicated specifically to learn the mechanisms of olfactory system damage in CKD are very limited, but it is possible that olfactory impairment is linked to neuronal damage associated with renal failure (Fig. 1). Alterations in olfactory function are a non-invasive biomarker of neurological dysfunction, as uraemic toxins can damage the integrity of the olfactory epithelium, the olfactory bulb and central areas for central processing of smell. In addition to the accumulation of uraemic toxins, other factors causing neuronal damage are oxidative stress, neuroinflammation and blood-brain barrier abnormalities.
Also capable of affecting olfactory function are the well-described changes and decrease in cerebral blood flow in patients with CKD, involving thickening of the arteriolar intimal layer, endothelial dysfunction, vascular calcification and dysfunction in the autoregulation of blood vessels.16
TreatmentChoosing the right dialysis and improving clearance of uraemic toxins are part of treatment to improve the symptoms associated with uraemic neuropathy. However, the reports on improvement of the olfactory system with dialysis treatment are the subject of debate, as not all studies have demonstrated post-dialysis improvement.12 Moreover, it is not known whether or not the dialysis modality (HD, PD) has a positive effect on patients’ olfactory function.10,13
Some studies have shown that renal transplant recipients recover olfactory function to a similar degree to that of the control groups; this is due to the high plasticity and capacity for recovery of the mucosa and olfactory neurons.10 One of the experimental treatments proposed to improve the function of the olfactory mucosa is theophylline. Theophylline is a phosphodiesterase activator which increases the intracellular level of cyclic adenosine monophosphate (cAMP) and induces activation of membrane transporters in conjunction with activation of V-ATPase. A small study in seven patients with CKD on HD and olfactory deficit demonstrated the utility of intranasal theophylline in correcting respiratory tract disorders in 70% of these patients.15
Function of olfactory receptors and the kidney (the smell of the kidney)Olfactory receptors are G protein-coupled receptors located in the cilia of sensory neurons and they exhibit great sensitivity for various volatile odorants. Because the renal epithelium is in continuous contact with a variety of chemicals, the kidney is an ideal organ to take advantage of the sensory functions of these receptors. Among other extra-olfactory organs, the presence of olfactory receptors has been demonstrated in the kidney, but the location in the nephron of the vast majority of these receptors is unknown.17 The majority of olfactory receptors are considered as “orphan receptors”, as their ligand remains unknown at present.18
Studies conducted by Pluznick et al.19 have shown recently that these receptors can play an important role in renin secretion, regulation of glomerular filtration and tubular reabsorption processes. The Olfr78 receptor is located in the afferent glomerular arteriole; this receptor is activated by short-chain fatty acids such as acetate and propionate, and probably regulates renin-mediated blood pressure; Olfr78-/- mice have low renin concentrations and normal blood pressure. Short-chain fatty acids are synthesised by the intestinal microbiota, and functional analyses have shown that the decrease in short-chain fatty acid-producing bacteria, with a decrease in acetate levels, is associated with the development of hypertension, meaning that this class of receptors could be related to changes in blood pressure mediated by the intestinal microbiota.20 The structurally related agonists OR51E1 and OR11H7 activate adenylate cyclase and increase intracellular calcium flow in the macula densa. Adenylate cyclase 3 [AC3] is an isoform of adenylate cyclase essential in olfactory signalling pathways, as AC3-/- knock-out mice lack olfactory ability. The AC3 kidney co-localisation with G-protein-coupled receptors in the macula densa and distal tubule is interesting. AC3-/- knock-out mice do not show changes in their blood pressure, but they do have a decrease in plasma renin concentration.21
Other olfactory receptors, such as the Olfr1393 have been shown to play an important role in the regulation of urinary glucose excretion, as they regulate the expression of SGLT 1 in the proximal tubule. Olfr1393-/- knock-out mice have reduced tubular expression of SGLT-2. This generates euglycaemic glycosuria22 and, in these mice with diabetes, a decrease in the progression of renal damage has been demonstrated.23
ConclusionOlfactory deficits are common in the population with CKD, but much of the pathophysiology involved remains unknown. The role of olfactory perception in food intake is widely known, so deterioration in this function could lead to poor patient nutrition. There is no established treatment at present to improve olfactory function in patients with CKD, but renal transplantation reverses the deficits. Olfactory receptors are also located in the kidney and have functions related to blood pressure control and glucose excretion.
- •
Alterations in the olfactory function of patients with CKD in pre-dialysis, PD and HD are common, although little recognised.
- •
The tests most widely used for assessing olfactory function are odour threshold, discrimination and identification tests.
- •
One of the most important consequences of olfactory deficits is their association with the patient's nutritional status.
- •
The effect of HD on smell is still not fully understood, as not all studies have shown correction of the deficit with treatment.
- •
Renal transplantation corrects dysosmia.
- •
Olfactory receptors located in the kidney have functions related to blood pressure control and glucose excretion.
The authors have no conflicts of interest to declare.
The authors would like to thank Olga Cuenca, MSc, for reviewing the manuscript.
Please cite this article as: Robles-Osorio ML, Corona R, Morales T, Sabath E. Enfermedad renal crónica y olfato. Nefrologia. 2020;40:120–125.