Immunocomplex-mediated membranoproliferative glomerulonephritis (MPGN) constitutes a histological pattern of glomerular damage produced by different diseases. Both the increase of immunocomplex production by autoimmune diseases or infections and the decrease of hepatic clearance of immunocomplexes can produce MPGN. This last mechanism is less frequent and would be the mechanism whereby a portosystemic shunt could produce MPGN.1
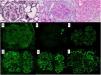
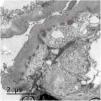
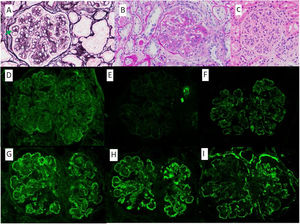
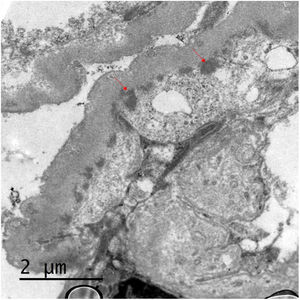
We present the case of a 53-year-old woman with a history of HIV on antiretroviral treatment, Liver cirrhosis secondary to HCV (diagnosed in 2008 and treated in 2016, with subsequent sustained viral response) without portal hypertension, and arterial hypertension with normal renal function. A study of nephrotic proteinuria is initiated upon finding in a routine test a proteinuria of 4076mg/g creatinine with albuminuria of 3662mg/g creatinine, microhematuria, hypercholesterolemia (263mg/dl), without hypoalbuminemia and without nephrotic syndrome. Immunological study was normal except for positive ANA at 1/160, decreased C4 (10.7mg/dl) and moderate elevation of rheumatoid factor (with negative cryoglobulins). At that time HIV and HCV viral loads remained undetectable, and as part of the study a contrast-enhanced scan was requested in which a shunt between the superior mesenteric vein and the right gonadal vein was observed. Having these findings, it was decided to perform a renal biopsy in which a diffuse lesion with hypercellular glomeruli was observed, with accentuation of the lobular pattern and presence of double contours were identified using the the PAS technique (Fig. 1). Likewise, there were subendothelial deposits, as well as occlusion of the capillary lumens. Immunofluorescence shows diffuse and generalized deposits of IgA (+), IgG (+++), IgM (++), C3 (+++), Kappa light chains (+) and Lambda light chains (++) in capillary loops and at mesangial level in a focal manner with granular appearance (Fig. 1). Electron microscopy shows basement membranes with frequent electrodense, unstructured deposits in the subepithelial side of the basement membrane with reduplication of the basement membrane, in addition to extensive pedicellar fusion (Fig. 2).
A) Methenamine silver, ×40: glomerulus with increased mesangial matrix and focal double contours (arrow). B) PAS, ×40: glomerulus with presence of double contours and occlusion of capillary lumens. C) HE, ×40: glomerulus with accentuation of the lobular pattern and increased endocapillary cellularity. Mesangial and capillary loops granular deposits of IgG +++ (D), IgA ++ (E), IgM ++ (F), Kappa ++ (G), Lambda ++ (H) and C3 +++ (I).
The patient was diagnosed of MPGN likely related to portosystemic shunt, so she was scheduled for embolization. A first embolization was performed in July 2020, which was partially effective, and the second one in June 2021 was completely effective. Thereafter, the values of C4 and cholesterol became normal, and the proteinuria a decrease to 88mg/g creatinine in her last follow up (Table 1).
Evolution of laboratory data.
| 06/2020 | 10/2020 | 12/2020 | 02/2021 | 07/2021 | 09/2021 | |
|---|---|---|---|---|---|---|
| Cholesterol (mg/dl) | 263 | 225 | 219 | 176 | 190 | 185 |
| Proteinuria (mg/g) | 4076 | 2173 | 985 | 615 | 529 | 88 |
| Albuminuria (mg/g) | 3662 | 1388 | 592 | 419 | 47 | |
| C4 (mg/dl) | 10.7 | 13 | 14.4 | 19 | ||
| Treatments | First partially effective embolization in August | Spironolactone | Effective embolization | |||
| 25mg |
Portosystemic shunts can produce MPGN both in patients with cirrhosis treated with transyugular intrahepatic shunts and without cirrhosis with congenital shunts.2–4 The underlying pathophysiological mechanism seems to be related to Kupffer cells; these cells are responsible for the clearance of immunocomplexes3; in circumstances in which their function is diminished (either due to cirrhosis or decreased portal flow), resulting in a systemic increase in circulating immunocomplexes that would be deposited at the renal level.
Regarding the histological changes, the MPGN secondary to shunts can produce deposits of C3, C4, IgM, IgG, C1q with predominance of IgA.2,5 Furthermore, the dominance of IgA deposits seems to depend on the magnitude of the shunt, being greater in those cases in which the shunt ratio is lower4; this seems to be related to the fact that IgA2 comes fundamentally from the gastrointestinal tract, and when a shunt occurs, these immunocomplexes would pass into the systemic circulation.1 In our case, IgA deposition was not dominant, which could be due to the high shunt ratio. Furthermore, we have observed by immunofluorescence that in addition to IgA, IgG, IgM and C3 deposits, we observed Kappa and Lambda light chains, a fact that had not been described previously in the literature.
Finally, the treatment of choice seems to be shunt embolization; in many cases this results in normalization of complement levels and hypoalbuminemia, as well as improvement of proteinuria and hepatic encephalopathy.4 In our case, after shunt embolization, it was observed a normalization of complement and hypercholesterolemia, as well as a marked improvement in proteinuria.