The SARS-CoV-2 pandemic has been the most devastating worldwide health, social and economic crisis in recent years. In the midst of the pandemic products were being promoted for the prevention and treatment of this coronavirus. Some of these products included chlorine dioxide or sodium chlorite, also known as Miracle Mineral Solution (MMS).1
Chlorine dioxide is a potent oxidising agent which rapidly dissociates in biological tissues producing its active agent, sodium chlorite.2
There is currently no scientific evidence to accredit safety or efficacy in the use of this substance and its derivatives against SARS-CoV-2. Furthermore, since 2010, different health authorities, such as the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency for Medicines and Medical Devices], the Food and Drug Administration (FDA), the Pan American Health Organisation (PAHO) and the Therapeutic Good Administration (TGA), have warned of the severe side effects related to the consumption of sodium chlorite.3,4
We present here the first case to be described in the literature of cerebral salt-wasting syndrome manifesting with severe hyponatraemia as an adverse reaction to chlorine dioxide consumption.
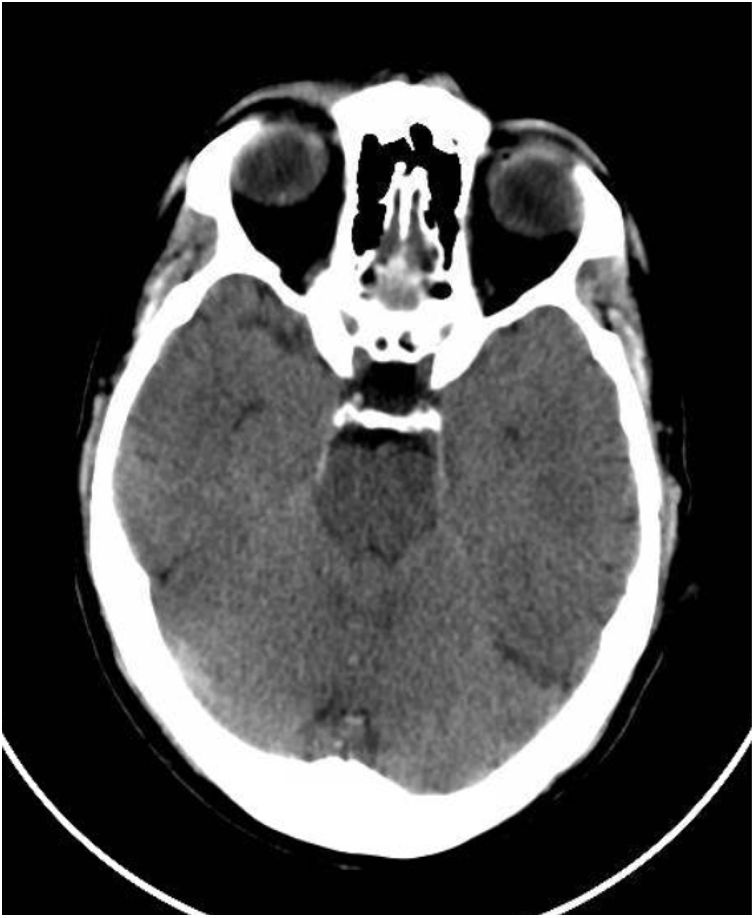
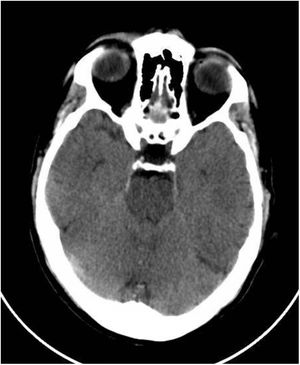
This was a 61-year-old male with no previous medical history, not vaccinated against SARS-CoV-2, who of his own free will began to consume chlorine dioxide daily in the belief that it would prevent the infection. After two weeks he developed gradual-onset encephalopathy symptoms with bradypsychia, derealisation, irritability and anxiety. Physical examination revealed dehydration of skin and mucosa. Brain computed tomography (CT) revealed cerebral oedema and idiopathic intracranial hypertension (Fig. 1). Fundus examination and lumbar puncture were normal. Blood tests showed: sodium 112 mEq/l; chlorine 77 mEq/l; plasma osmolarity 230 mOsm/kg; and uric acid 2.2 mg/dl; and fractional excretion of uric acid (FEUa) on admission was 15.4% and after 72 h with normalisation of natraemia, 11.4%. Venous blood gases: pH 7.42, bicarbonate (HCO3) 21 mmol/l, CO2 42 mmHg. No other findings of note. Urinalysis: sodium 72 mEq/l, potassium 30 mEq/l, chlorine 55 mEq/l, uric acid 15.4 mg/dl and osmolarity 224 mOsm/kg. The chlorine dioxide was immediately discontinued and treatment was started for water and electrolyte replacement progressively according to sodium deficit, with gradual restoration of neurological status and return to normal of analytical parameters (Table 1). A differential diagnosis of “other possible causes of hyponatraemia” was made.
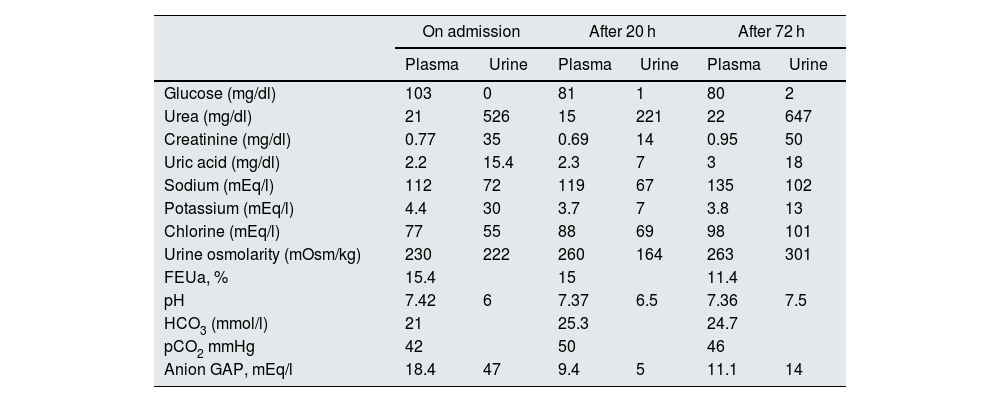
Changes over time in laboratory parameters.
| On admission | After 20 h | After 72 h | ||||
|---|---|---|---|---|---|---|
| Plasma | Urine | Plasma | Urine | Plasma | Urine | |
| Glucose (mg/dl) | 103 | 0 | 81 | 1 | 80 | 2 |
| Urea (mg/dl) | 21 | 526 | 15 | 221 | 22 | 647 |
| Creatinine (mg/dl) | 0.77 | 35 | 0.69 | 14 | 0.95 | 50 |
| Uric acid (mg/dl) | 2.2 | 15.4 | 2.3 | 7 | 3 | 18 |
| Sodium (mEq/l) | 112 | 72 | 119 | 67 | 135 | 102 |
| Potassium (mEq/l) | 4.4 | 30 | 3.7 | 7 | 3.8 | 13 |
| Chlorine (mEq/l) | 77 | 55 | 88 | 69 | 98 | 101 |
| Urine osmolarity (mOsm/kg) | 230 | 222 | 260 | 164 | 263 | 301 |
| FEUa, % | 15.4 | 15 | 11.4 | |||
| pH | 7.42 | 6 | 7.37 | 6.5 | 7.36 | 7.5 |
| HCO3 (mmol/l) | 21 | 25.3 | 24.7 | |||
| pCO2 mmHg | 42 | 50 | 46 | |||
| Anion GAP, mEq/l | 18.4 | 47 | 9.4 | 5 | 11.1 | 14 |
FEUa: fractional excretion of uric acid; HCO3: bicarbonate; pCO2: partial pressure of carbon dioxide; pH: hydrogen potential.
Consumption of chlorine dioxide increased exponentially during the pandemic due to the spread of misinformation in the media and social media about its possible prophylactic effect on the development of SARS-CoV-2 infection. The most common adverse reactions to chlorine dioxide are respiratory failure, heart rhythm disturbances, acute liver failure, hypotension, gastrointestinal disturbances and acute kidney injury.5,6 Severe gastrointestinal disorders have been reported in association with the use of this substance which could cause hyponatraemia, secondary to the patient's renal loss of sodium. No cases of cerebral salt-wasting syndrome following ingestion of this substance have been reported in the literature to date.
In the case described, a presumptive diagnosis of cerebral salt-wasting syndrome was established, characterised by hyponatraemia (plasma sodium less than 135 mEq/l), hypotonic (plasma osmolarity <280 mOsm/kg), hypovolaemic (presence of signs of dehydration), with renal sodium losses with urinary sodium >40 mEq/l and high urinary osmolarity (>100 mOsm/kg) accompanied by hypouricaemia (uric acid less than 4 mg/dl) and high fractional excretion of uric acid (FEUa) (>10–12%). The severity of the condition was determined by the development of neurological symptoms of slow and progressive onset, with organic brain damage being ruled out by imaging and lumbar puncture. The above clinical and analytical data, the absence of diuretic treatment and the within-normal plasma levels of urea, creatinine, cortisol and aldosterone therefore suggested a diagnosis of cerebral salt-wasting syndrome.7
The pathogenesis of this syndrome is poorly understood and it is usually transient, resolving completely within weeks, making the differential diagnosis with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) essential.8 The FEUa helps in the differential diagnosis of salt-wasting syndrome with SIADH, since in salt wasting syndrome, as in our case, the FEUa is greater than 11% and is not corrected after return to normal of blood sodium levels.9
Applying the causality algorithm of the Spanish Pharmacovigilance System in this case, it is likely that the condition was an adverse reaction related to chlorine dioxide, as there was a clear time sequence between ingestion and onset of symptoms, and complete resolution after discontinuation of the agent. Although how chlorine dioxide may cause hyponatraemia is not fully understood, we could postulate as a theory that, as its active agent is sodium dioxide, a renal reaction to an excess of the active agent, sodium chlorite, would cause an increase in urinary sodium losses, leading over time to subacute hyponatraemia, as in the case we describe here.
We believe our case is relevant because of the need to raise public awareness of the various adverse effects related to this substance, and the lack of evidence to accredit its use in the prevention of SARS-CoV-2 infection, and thus dissuade people from taking it.
FundingNo funding has been received from any funding body.