Antecedentes: El efecto de cinacalcet en pacientes con hiperparatiroidismo secundario persistente (HPTS) tras el trasplante renal (TR) ha sido descrito principalmente en pacientes con hipercalcemia secundaria. Objetivos: Nuestro objetivo fue evaluar el efecto a largo plazo de cinacalcet en pacientes con TR y HPTS normocalcémico. Métodos: Estudio multicéntrico, observacional, retrospectivo, de un año, que incluyó receptores renales con HPTS (hormona paratiroidea intacta [PTHi] > 120 pg/ml) y niveles de calcio dentro de la normalidad (8,4-10,2 mg/dl) que iniciaron cinacalcet en la práctica clínica. Resultados: Se incluyeron 32 pacientes con una edad media (desviación estándar [DE]) de 54 (11) años, 56 % varones. El tratamiento con cinacalcet se inició una mediana de 16 meses después del TR (dosis mediana de 30 mg/día). Los niveles de PTHi disminuyeron desde una mediana (P25, P75) de 364 (220, 531) pg/ml al inicio del estudio a 187 (98, 320) a los 6 meses (reducción del 48,6 %, p = 0,001) y a 145 (91, 195) a los 12 meses (reducción del 60,2 %, p = 0,001), sin cambios en los niveles de calcio y fósforo (p = 0,214 y p = 0,216, respectivamente). No se observaron cambios en la función renal ni en los niveles de fármacos anticalcineurínicos. El 3,1 % de los pacientes interrumpió cinacalcet debido a intolerancia, y el 6,2 %, debido a falta de eficacia. Conclusiones: En pacientes con HPTS normocalcémico tras el TR, cinacalcet mejora el control de los valores séricos de PTH sin provocar cambios en la calcemia o fosfatemia ni en la función renal. Cinacalcet mostró una buena tolerabilidad.
Background: The effect of cinacalcet in patients with persistent secondary hyperparathyroidism (SHPT) after kidney transplantation (RT) has mainly been reported in patients with secondary hypercalcaemia. Objectives: Our objective was to assess the long-term effect of cinacalcet on patients with a RT and normocalcaemic SHPT. Methods: A one-year multicentre, observational, retrospective study that included kidney recipients with SHPT (intact parathyroid hormone [iPTH] >120pg/ml) and calcium levels within the normal range (8.4-10.2mg/dl). Patients began treatment with cinacalcet in clinical practice. Results: 32 patients with a mean age (standard deviation [SD]) of 54 (11) years, 56% male, were included in the study. Treatment with cinacalcet began a median of 16 months after RT (median dose of 30mg/day). Levels of iPTH decreased from a median (P25, P75) of 364 (220, 531) pg/ml at the start of the study to 187 (98, 320) after 6 months (48.6% reduction, P=.001) and to 145 (91, 195) after 12 months (60.2% reduction, P=.001), without there being changes in calcium and phosphorus levels (P=.214 and P=.216, respectively). No changes were observed in kidney function or anti-calcineuric drug levels. 3.1% of patients discontinued cinacalcet due to intolerance and 6.2% due to a lack of efficacy. Conclusions: In patients with normocalcaemic SHPT after RT, cinacalcet improves the control of serum PTH values without causing changes to calcaemia, phosphataemia or kidney function. Cinacalcet showed good tolerability.
INTRODUCTION
Secondary hyperparathyroidism (SHPT) that persists after kidney transplantation is associated with a high bone turnover rate and an increased risk of fractures.1 We do not have a full picture of the causes of SHPT recurrence, but some associated factors have been identified, such as hyperplasia persistence in the parathyroid gland with independent production of the parathyroid hormone (PTH), its slow regression or kidney dysfunction.2 Post-transplant hyperparathyroidism does not usually resolve spontaneously and its persistence ultimately depends on graft function and the severity of SHPT before transplantation.3-5
Cinacalcet (Sensipar®/Mimpara®) is a second-generation calcimimetic agent administered orally that binds to calcium-sensing receptors in a different location to calcium (allosteric agonist), which increases parathyroid cell sensitivity to extracellular calcium and slows down secretion and production of PTH. Cinacalcet effectively reduces serum PTH levels and corrects serum phosphorus and calcium levels in patients with chronic renal failure on dialysis.6-8 In patients with SHPT after kidney transplantation, the effect of cinacalcet has been assessed, but mainly in patients with high serum calcium levels associated with elevated PTH.9-28 These studies showed a sustained reduction in PTH levels of approximately 50%, 6 months after treatment was started.28 In Spain, cinacalcet is currently the first line of treatment for hypercalcaemic SHPT following kidney transplantation29 and a clinical trial is being carried out to support this indication.30 Despite the lack of experience in the literature, cinacalcet is also administered to some patients with high PTH levels who do not have concomitant hypercalcaemia. To our knowledge, only one previous study has analysed the effect of cinacalcet in patients with a kidney transplant with normocalcaemic SHPT.23 The objective of this study was to assess the long-term effect of cinacalcet in patients with normocalcaemic SHPT after kidney transplantation, who were followed up for one year.
PATIENTS AND METHODS
A multicentre, observational, retrospective study was carried out in 17 Spanish kidney transplant units and data were collected from April to November 2011. The main inclusion criteria were: kidney transplant recipients ≥ 18 years of age, with persistent SHPT following kidney transplantation and calcium serum values within the normal range (defined as total corrected serum calcium ≥8.4 and ≤10.2mg/dl), who began treatment with cinacalcet in clinical practice before 31 July 2009 (independently of the time elapsed between transplantation and the start of treatment), with available data for intact parathyroid hormone (iPTH), calcium and phosphorus values in serum at baseline (before introducing cinacalcet) and in at least one post-baseline evaluation. To avoid selection bias, the clinical histories of all patients in each hospital who had undergone kidney transplantation and had begun treatment with cinacalcet were reviewed. All patients who met the selection criteria were included in the study. Before starting treatment, the patients were required to sign their informed consent to receive cinacalcet, as well as requiring administrative authorisation. The study adhered to the Declaration of Helsinki (2000) and the Declaration of Istanbul (2008). The study protocol was approved by the ethics committees of each participating hospital.
Baseline was considered to be the date on which cinacalcet was introduced (month 0). The other data collection dates for the study were selected in accordance with the clinical practices of participating hospitals: months 1, 3, 6 and 12 or until 31 January 2010. Data were recorded using an online electronic logbook. To guarantee quality, the database contained logical controls and 100% of the information was verified by external monitors. During this revision, consistency, lost data and apparent data discrepancies were checked. The main variables were iPTH, phosphorus and calcium values in serum (6 months after introducing cinacalcet). We also recorded demographic and clinical characteristics, laboratory data, concomitant treatment and cinacalcet discontinuation during the follow-up period. Only adverse reactions causing treatment discontinuation were recorded.
Statistical analysis
Categorical variables were summarised using frequencies and percentages, and continuous variables were summarised using means and standard deviation (SD) or standard error, or medians and 25th and 75th percentiles (P25, P75). Statistical analysis was only based on the data observed, with the exception of the main results after 6 months, in which lost data were replaced with the latest measurement available. Changes to baseline values in post-baseline visits were evaluated using paired Student’s t-tests or Wilcoxon signed-rank tests. Statistical analysis was carried out using the SAS® version 8.2 software (SAS Institute, Cary, NC, USA).
RESULTS
Study population
We collected the data of 32 patients who started cinacalcet due to normocalcaemic SHPT. During follow-up, 3 patients (9.3%) discontinued cinacalcet: 2 (6.2%) due to a lack of efficacy and 1 (3.1%) due to tolerability problems.
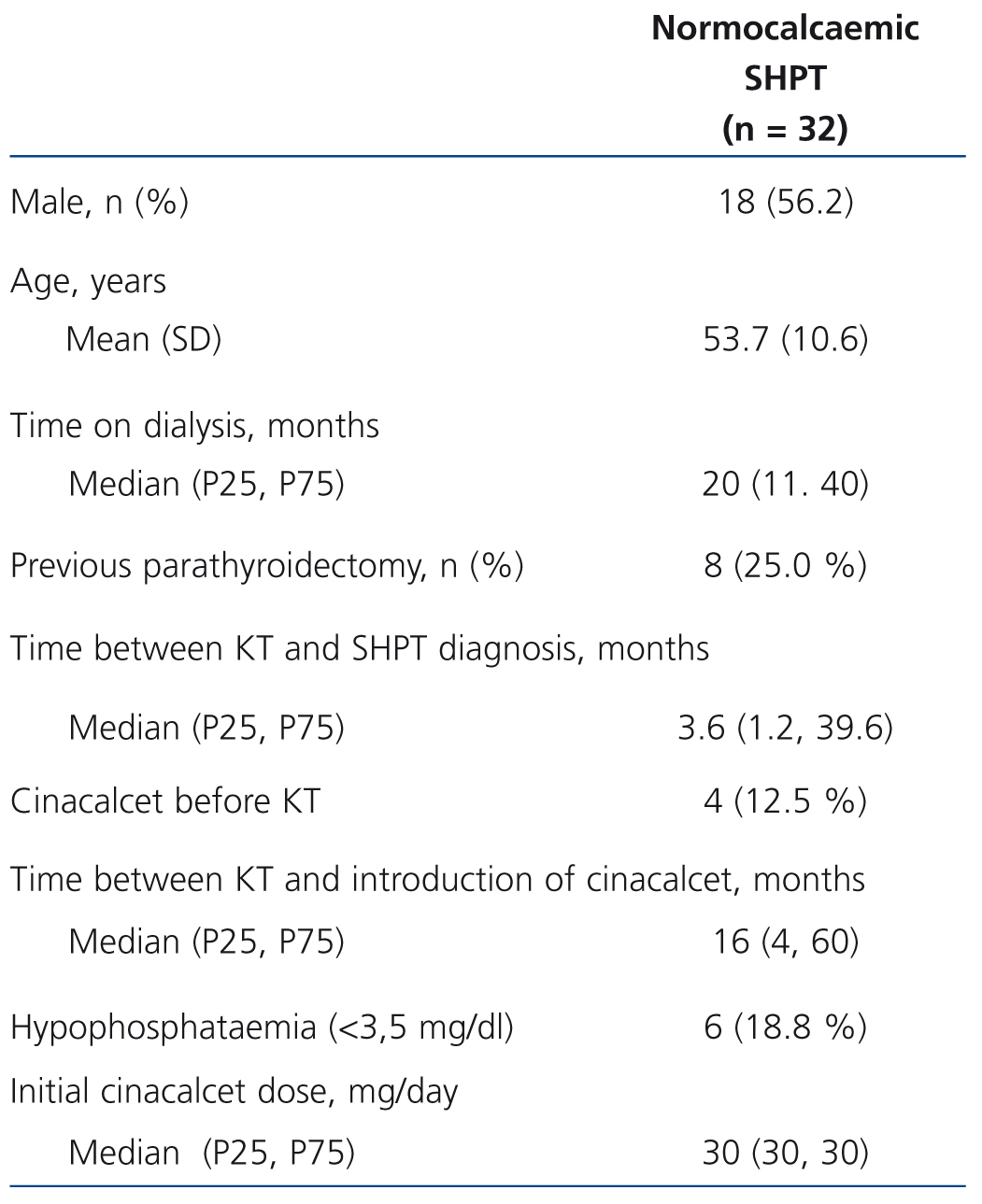
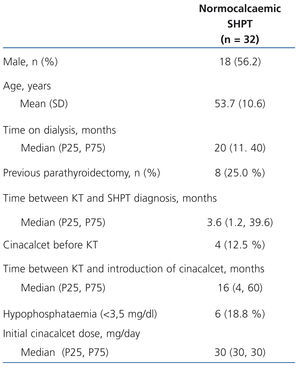
Table 1 displays the main population characteristics on cinacalcet introduction. 18.8% of patients had hypophosphataemia at the start of the study and none had hyperphosphataemia. The mean follow-up time (SD) was 19 (10) months. The study included a total of 51 patients-year of observation. Time until SHPT diagnosis following kidney transplantation was less than 2 months in 37.9% of patients and more than 3 years in 27.6%. The median time from kidney transplantation to cinacalcet introduction in the whole sample was 16 months. During follow-up, a slight increase in the mean dose (SD) of cinacalcet was observed: from 30 (0) mg/day in the baseline visit (median [range]: 30 [30-30] mg/day, compared to 42 (21) mg/day after 12 months (median [range]: 30 [10-90] mg/day). Most patients were treated with an immunosuppressive regimen that included calcineurin inhibitors (tacrolimus, 75.0%, ciclosporin, 12.5%), in combination with mycophenolate mofetil or mycophenolic acid in 71.9% of cases and prednisone in 54.8%. The other immunosuppressants used were sirolimus and everolimus (9.4% and 3.1% respectively).
Effect of cinacalcet
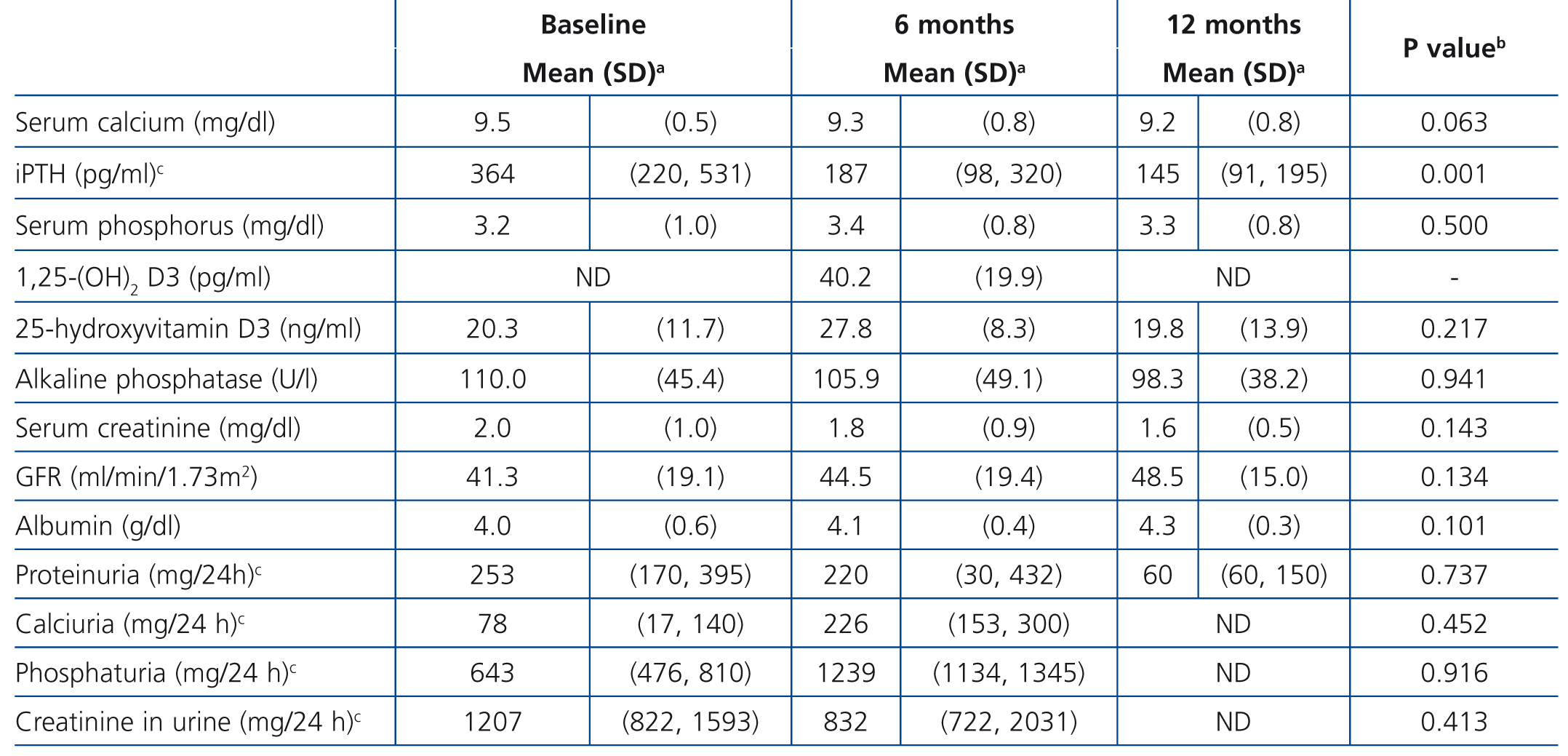
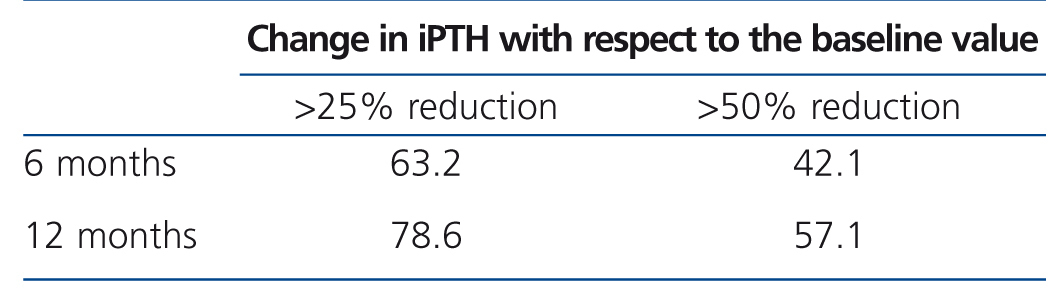
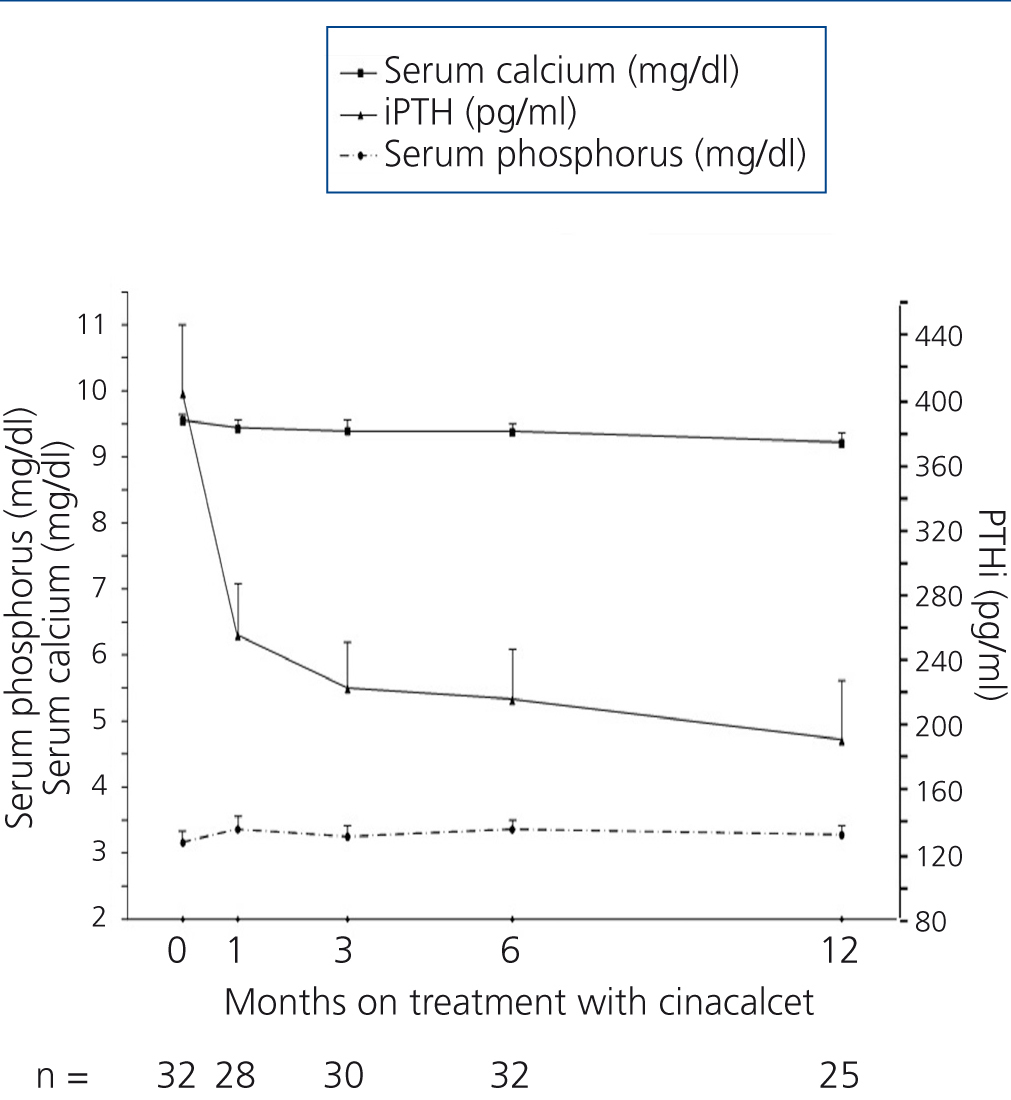
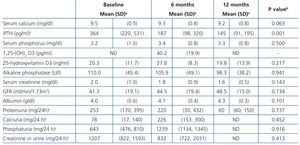
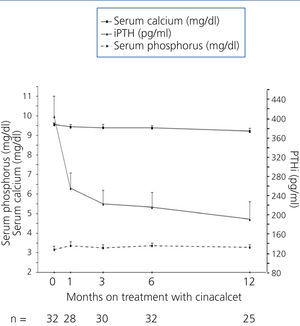
Table 2 and Figure 1 display progression of biochemical values over time. After cinacalcet was introduced, no significant changes were observed in serum calcium and phosphorus during follow-up (p=.063 and p=.500 after 12 months compared to baseline, respectively), however, there was a downward trend for calcaemia. A significant 46.1% decrease was observed in iPTH levels during the first month (from a median of 364 to 196pg/ml, P<.0001), which continued to decrease until the 12th month (Table 2 and Figure 1). After 6 months, median levels had decreased by 48.6% (p<.0001) and, after 12 months, by 60.2% (p=.001). Table 3 displays the percentages of patients who achieved a reduction of more than 25% and 50% in iPTH levels with respect to their baseline value, at different points in time. After 6 months, 28.1% of patients had achieved serum PTH values within the recommended levels for their kidney function and this figure remained the same after 12 months (28.6%). No parathyroidectomies were performed during follow-up.
No significant changes in albumin levels, glomerular filtration rate or creatinine levels were observed over time.
Major changes or changes in the percentage of patients treated with vitamin D supplements or analogues were not observed over time (data not displayed). Throughout the study, 19.3% of patients received native vitamin D and 51.6% received vitamin D analogues.
Safety
One patient (3.1%) discontinued treatment with cinacalcet due to a non-severe adverse reaction (gastrointestinal discomfort).
DISCUSSION
To date, this study is the second cohort published on kidney transplant recipients with normocalcaemic SHPT treated with cinacalcet in clinical practice. The sociodemographic characteristics and baseline kidney function were comparable to data from other kidney transplant recipient series from the same years.31,32 Our results show that cinacalcet causes a sustained reduction in iPTH levels without inducing hypocalcaemia or hyperphosphataemia. To date, there has only been one previous retrospective study, lasting 12 months, that analysed the effect of cinacalcet in patients with a kidney transplant and SHPT without hypercalcaemia.23 The relative reduction in iPTH levels after 12 months was similar in the two cohorts (60% compared to 49% in the study by Gómez Marqués et al.23). Notably, more than two thirds of patients displayed a good response to treatment with cinacalcet in terms of iPTH reduction and almost a third remained within the control objectives established by the Spanish Society of Nephrology recommendations.29 These results are very different from those observed in patients with tertiary hyperparathyroidism and hypercalcaemia, in which calcaemia control was achieved with a very modest effect on serum PTH values.6,33
With regard to the effect on serum calcium values, although there was a slight reduction in the first few months, a significant effect was not observed, whereas in the study by Gómez Marqués et al. they detected a more significant reduction from 9.6 to 8.9mg/dl in one year.23 The baseline calcium level was similar in the two cohorts, and in the study by Gómez Marqués et al., more patients received vitamin D analogues (79% compared to 52% in our cohort). However, 24% of their patients had hypocalcaemia (<8 mg/dl). In our study, only 3 patients (9.4%) displayed a calcium level <8mg/dl during follow-up. Two received calcitriol and in only one case, cinacalcet was discontinued due to persistent hypocalcaemia. No abnormal serum phosphorus values were observed in either study.
The modest effect on calcaemia and no effect on phosphataemia is not observed in SHPT patients with chronic kidney disease not on dialysis, in whom a significant reduction in serum calcium values and a significant increase in serum phosphorus values is usually observed.34-37 Several factors probably influence this, amongst which we could highlight that serum PTH values are lower in patients with a kidney transplant, and in particular that these patients have tertiary hyperparathyroidism, and as such, they are more resistant to cinacalcet treatment.
The incidence of gastrointestinal problems that led to treatment discontinuation was low and is comparable to that of other studies carried out previously in haemodialysis patients.37 Perhaps the fact that doses are low and the dose increase was moderate and gradual had a positive effect on the good tolerance observed in our series. No interaction was observed between immunosuppressant drug use and tolerance to cinacalcet, and no abnormalities in blood calcineurin inhibitor levels were reported.12,15-18,23,38 There was a progressive decrease in tacrolimus levels over time, comparable to that observed in other patients not treated with cinacalcet (data not displayed). There were no changes in kidney function, which is consistent with previous studies in patients with hypercalcaemic SHPT.12,15-18,23,38 A trend towards an improvement in the glomerular filtration rate was even observed, accompanied by reduced proteinuria and an increase in serum albumin, although these changes did not achieve statistical significance.
The main limitations of our study were the retrospective collection of data, which did not allow selection or information bias to be excluded, as well as the small sample size.
In conclusion, in normocalcaemic SHPT patients who have received a kidney transplant, cinacalcet improves control of serum PTH values without causing changes in calcaemia, phosphataemia or kidney function. Cinacalcet displayed good tolerability.
SOURCES OF FINANCING
This study was financed in part by a grant from Amgen. Amgen did not participate in the study design, the collection, analysis or interpretation of data or in the decision to send its results for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Acknowledgements
This study was carried out under the auspices of the Spanish Society of Nephrology. Data monitoring and statistical analysis were carried out by TFS-Develop. Dr Neus Valveny (TFS-Develop) assisted in the scientific writing.
Table 1. Baseline characteristics of patients with persistent normocalcaemic secondary hyperparathyroidism after kidney transplantation who received cinacalcet
Table 2. Laboratory data progression during follow-up in patients with normocalcaemic secondary hyperparathyroidism
Table 3. Percentage of patients with specified reduction in intact parathyroid hormone at each point in time.
Figure 1. Progression of serum calcium, phosphorus and parathyroid hormone values after introduction of cinacalcet.