To the Editor,
Microscopic polyangiitis (MPA) is defined as a non granulomatous, anti-neutrophil cytoplasmic antibodies (ANCA)-positive systemic necrotising vasculitis which affects small vessels. Kidneys and lungs are the most frequently affected organs.1 Pulmonary bleeding in MPA is usually acute or even fulminant.2,3 Pulmonary bleeding as part of chronic MPA is not described as often. Autoimmune thyroiditis is associated with glomerulopathies, especially with membranous nephropathy.4
We describe a patient with chronic pulmonary bleeding as a sign of MPA; the examination also showed that she presented with autoimmune thyroiditis.
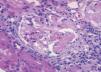
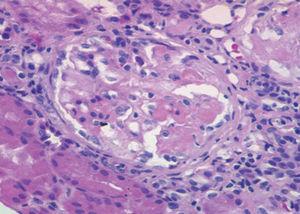
A 54-year-old woman under examination for a year and a half in another hospital for iron deficiency anaemia of several years. She was being treated with orally-administered iron and needed transfusions. They found that she had renal failure, proteinuria and microhaematuria during this examination, and she was referred to us. Patient history: arterial hypertension for a year. Clinically, she presented with important asthenia and medium-effort dyspnoea. Physical examination: she did not present with fever, blood pressure was 140/96mm Hg, and had no oedemas on lower limbs. The analytical study showed: haemoglobin: 8.6g/dl; iron: 33.5µg/dl; total iron-binding capacity (TIBC): 264µg/dl; ferritin: 137ng/ml; urea: 67mg/dl; creatinine: 1.50mg/dl; estimated glomerular filtration rate (eGFR) (according to Modification of Diet in Renal Disease [MDRD4]): 38.46ml/min/1.73m2; uric acid: 7.19mg/dl; total cholesterol: 233mg/dl (LDL: 163; HDL: 33.7); triglycerides: 178mg/dl. Normal/negative immunoglobulins, antinuclear antibodies (ANA), anti-DNA, rheumatoid factor (RF), anti-glomerular basement membrane antibody treatment (anti-MBG) and anti-transglutaminase antibodies (ATA); positive myeloperoxidase (MPO)-ANCA, negative PR3-ANCA. High resolution electrophoresis (HRE) of serum: no monoclonal band. Thyroid-stimulating hormone (TSH): 326mIU/l (0.350-4.940), free thyroxine (T4): 0.11ng/dl (0.7-1.48); anti-thyroid peroxidase antibodies (anti-TPO) >1000IU/ml. Negative Mantoux test. Negative hepatitis B surface antigens (HbsAg), hepatitis B anti-core antibodies (anti-HBc), hepatitis C antibodies (anti-HCV) and human immunodeficiency virus (HIV) tests. Cytomegalovirus (CMV) serology test and Epstein-Barr virus (EBV): previous exposure. Urine showed a proteinuria of 1.3g/24hr (moderately selective glomerular proteinuria), sediment with 60-100 red blood cells (30%-50% dysmorphic) and hyaline-granular casts. Urine culture: negative. Chest X-ray and electrocardiogram (ECG) were normal. Abdominal ultrasound: kidneys of a normal size with slight cortical thinning. Chest computerised tomography (CT) showed images of ‘ground-glass opacity’ on both sides in the middle and lower fields and a bronchoscopy with a bronchoalveolar lavage was indicated, showing 80% siderophores. The malignant cell cytology and cell cultures were negative. The patient was interviewed, revealing that she sporadically presented with a slight, dark-coloured expectoration. The renal biopsy (Figure 1) showed a focal and segmental necrotising glomerulonephritis, and approximately a third of the glomeruli were sclerotic, interstitial fibrosis and tubular atrophy in 30% of the cylinder. The immunofluorescence study showed slight deposits of IgM, C3 and C4 on capillary walls and the mesangium. Given the pulmonary bleeding, the positive ANCA, and the focal necrotising glomerulonephritis with scarce immune deposits, MPA was diagnosed, associated with autoimmune thyroiditis-induced hypothyroidism. Treatment with prednisone at 50mg/day was started for 8 weeks, gradually reducing the dosage, and six 750-mg cyclophosphamide boli, which was followed by mycophenolate mofetil at 500mg/8hr and levothyroxine at 75µg/day. At 6 months from diagnosis, the haemoglobin (Hb) was 12.6g/dl; creatinine: 1.4mg/dl; sediment: 10-20 red blood cells/field; ANCA were negative and thoracic CT did not show infiltrates.
Pulmonary bleeding symptoms include haemoptysis, coughing, chest pain, dyspnoea, anaemia and acute respiratory failure. Chronic evolution of pulmonary bleeding in MPA with non-specific symptoms and iron deficiency, as occurred with our patient, is less documented.2,5 However, in Lauque et al’s study, 28% of patients had symptoms for more than a year before the pulmonary bleeding was diagnosed.2
Autoimmune thyroiditis is associated with glomerulopathies, especially with membranous,4 membranoproliferative6 and IgA glomerulonephritis.7 A common pathogenic link is suggested given that the two autoimmune diseases are present at the same time.4 It is thought that thyroglobulin and thyroid peroxidase released by destroying the thyroid follicles would be deposited in the kidney and in situ immunocomplexes would be formed; depositing of circulating immunocomplexes seems less likely.8 In the case of autoimmune thyroiditis with ANCA vasculitis, other mechanisms could be involved, such as polyreactive antibody development and regulator and effector T cell alterations.8 With regards the antibodies, it is worth highlighting that myeloperoxidase shares a certain structural homology with thyroid peroxidase and that it could produce cross-over reactions,9 but other authors do not support this mechanism.10 Although the association between autoimmune thyroiditis and ANCA-positive vasculitis does not seem to be common, Tanaka et al9 found that 4 cases out of a series of 10 with ANCA-positive nephropathy had hypothyroidism, 2 of which were subclinical.
To conclude, it is important to consider that pulmonary bleeding in the MPA may evolve over a long period, with no marked haemoptysis or changes visible in the chest X-ray, being the cause of iron deficiency anaemia. For patients with ANCA vasculitis it is convenient to determine thyroid function and anti-TPO antibodies, and for those with autoimmune thyroiditis, the possibility that patients have an underlying nephropathy must be assessed.
Figure 1. Optical microscope. Glomerule with fibrinoid necrosis. HE stain, x20.