Hypertension (HT) is the second leading cause of kidney failure. In hypertensive patients with chronic kidney disease (CKD), blood pressure (BP) control is the most important intervention to minimise progression. For CKD diagnosis, standardised creatinine and estimated glomerular filtration rate (eGFR) testing by CKD-EPI is recommended.
ObjectivesTo describe the prevalence and factors associated with a moderate decrease in eGFR (by CKD-EPI) and BP control in subjects with HT.
MethodsCross-sectional descriptive study in subjects ≥60 years included in the SIDIAP plus database with hypertension and standardised serum creatinine and BP tests in the last 2 years. Exclusion criteria: eGFR<30, dialysis or kidney transplantation, prior cardiovascular disease, home care. Primary endpoint: eGFR by CKD-EPI formula. Covariates: demographic data, examination, cardiovascular risk factors, heart failure and auricular fibrillation diagnosis, and drugs (antihypertensive agents acting on renal function, antiplatelet and lipid lowering agents). BP control criteria: ≤130/80mmHg in individuals with albuminuria, ≤140/90 in all other subjects.
ResultsPrevalence of eGFR <60=18.8%. Associated factors: age, gender, heart failure, albumin/creatinine ratio, auricular fibrillation, smoking, dyslipidaemia, diabetes and obesity. BP control: 66.14 and 63.24% in eGFR≥60 and eGFR<60, respectively (p<0.05). Exposure to drugs was higher in eGFR<60.
ConclusionOne in 5 hypertensive patients without cardiovascular disease ≥60 years in primary care presented with a moderate decrease in eGFR. In addition to age and sex, albuminuria and heart failure were the main associated factors. Despite the increased exposure to drugs, BP control was lower in CKD.
La hipertensión arterial (HTA) es la segunda causa de insuficiencia renal. En hipertensos con enfermedad renal crónica (ERC) el control de la presión arterial (PA) es la intervención más importante para minimizar la progresión. Para el diagnóstico de ERC se recomienda la determinación estandarizada de creatinina y filtrado glomerular estimado (FGe) según CKD-EPI.
ObjetivosDescribir la prevalencia y los factores asociados a la disminución moderada del FGe (según CKD-EPI) y el control de PA en individuos con HTA.
MétodosEstudio descriptivo transversal en individuos ≥60 años incluidos en la base de datos SIDIAP plus con HTA y registro de creatinina sérica estandarizada y PA en últimos 2años. Criterios de exclusión: FGe<30, diálisis o trasplante renal, enfermedad cardiovascular previa, atención domiciliaria. Variable principal: FGe según CKD-EPI. Covariables: datos demográficos, exploración, factores de riesgo cardiovascular, diagnósticos de insuficiencia cardiaca y fibrilación auricular y fármacos (antihipertensivos con acción sobre función renal, antiagregantes, hipolipidemiantes). Criterio de control de la PA: ≤130/80mmHg en individuos con albuminuria, ≤140/90 en el resto.
ResultadosPrevalencia FGe<60: 18,8%. Factores asociados: edad, sexo, insuficiencia cardiaca, cociente albúmina/creatinina, fibrilación auricular, hábito tabáquico, dislipidemia, diabetes y obesidad. Control de la PA: 66,14 y 63,24% en FGe≥60 y FGe<60 respectivamente (p<0,05). La exposición a fármacos fue superior en FGe<60.
ConclusionesUno de cada 5hipertensos sin enfermedad cardiovascular ≥60 años en atención primaria presentó disminución moderada del FGe. Además de la edad y el sexo, la albuminuria y la insuficiencia cardiaca fueron los principales factores asociados. A pesar de la mayor exposición a fármacos, el control de la PA fue inferior en ERC.
Chronic kidney disease (CKD), defined as a reduction of the estimated glomerular filtration rate (eGFR) below 60mL/min/1.73m2 or the presence of kidney damage,1 is associated with an increased risk of cardiovascular morbidity and mortality and progression to end-stage renal disease (ESRD) in both the general population and in hypertensive patients.2–5 Deaths from CKD increased by 82% worldwide between 1990 and 2010; this is the third uppermost increase out of the 25 leading causes of death after HIV/AIDS (396%) and diabetes (93%).6
Hypertension (HTN) is the second most common cause of ESRD. The number of cases of ESRD with a primary diagnosis of HTN is increasing, especially in the individulas >45 years, this is a consequence of greater survival rates in kidney failure patients and longer life expectancy.
In hypertensive patients with CKD, blood pressure (BP) control is essential to minimise progression of CKD, reduce complications inherent to kidney failure and reduce the associated risk of cardiovascular disease.1,7,8 However, there is some debate about optimal values of BP. In the latest guidelines, the target BP of ≤130/80mmHg has been limited to individuals with albuminuria of 30–300mg/dL1 or overt albuminuria,7 while the target value of ≤140/90mmHg has been maintained for the rest and, in some cases8 for all hypertensive patients.
Current recommendations for the diagnosis of CKD include standardised determination of creatinine and eGFR using the CKD-EPI formula.1 The CKD-EPI formula is more accurate at higher values of eGFR than previous formulas, and many studies have shown that it provides a lower estimate of the prevalence of CKD and a better prognostic classification.9–11 In Spain there are no studies in hypertensive patients using the above criteria.
The objective of this study is to describe the prevalence and factors associated with a moderate decrease in eGFR (30–59 60mL/min/1.73m2) estimated by CKD-EPI and to assess the degree of BP control in relation to wheter there is a reduction in eGFR, following the most recent recommendations for hypertensive patients aged ≥60 years, with no history of cardiovascular disease, treated in Primary Care Facilitites. This study is part of a cohort study primarily aiming to quantify the risk of cardiovascular events associated with a moderate decrease in eGFR and the progression of eGFR deterioration in relation to the degree of BP control in hypertensive individuals.
Material and methodsThis is a cross-sectional descriptive study. The data were obtained from the Information System for the Development of Research in Primary Care (SIDIAP), which contains clinical information about patients treated at the 274 primary healthcare centres of the Catalan Institute of Health (ICS) in Catalonia, which provides services to 5,835,000 patients (80% of the population). To ensure the quality of the data, a selected database, SIDIAP plus12 was used which has shown to be valid and representative of the population for cardiovascular epidemiology studies.13
We selected individuals aged ≥60 years included in the SIDIAP plus database since January 1st 2011, with a coded diagnosis of HTN (CIE10 codes: I10. I15 and subcategories) in the electronic primary care records, with a minimum follow up of 2 years, with measurement of standardised serum creatinine determination and BP at least once in the preceding 2 years, and with a maximum interval of 6 months. Exclusion criteria were: a) CKD stages 4 and 5 (eGFR<30mL/min, dialysis or renal transplant); b) previous cardiovascular disease (myocardial infarction, angina, established or transient cerebrovascular accident, peripheral artery disease); and c) individuals included in a home care programme.
Renal function was assessed using CKD-EPI,9 without adjusting for race (not available), and KDIGO (Kidney Disease Improving Global Outcomes) classification.1 The last creatinine level and albumin/creatinine ratio (ACR), determined prior to 01/01/2011, were considered in each study subject. Covariables were: gender, age, rurality index (according to municipality of residence: urban if more than 10,000 inhabitants and population density >150inhabitants/km2, or rural if otherwise), socioeconomic deprivation index (MEDEA),14 smoking habit, systolic (SBP) and diastolic (DBP) blood pressure, weight and height, glucose, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, and coded diagnoses of heart failure (HF) and atrial fibrillation. As a criterion of contemporaneity, we considered the median of all determinations with a maximum interval of 6 months between them, as well as the median between the determinations and the relevant date of creatinine determination.
The presence of cardiovascular risk factors was defined as: a) diabetes mellitus (DM): codes E11, E12, E14 and subcategories (2 determinations of fasting blood glucose >126mg/dL or typical symptoms with random blood glucose >200mg/dL); b) hypercholesterolaemia: total cholesterol >250mg/dL or lipid-lowering drugs; c) smoking habit: smoker (daily consumption of one or more cigarettes during the last month, code F17), ex-smoker (more that 1 year with the diagnosis of smoker removed code Z72.0); and d) obesity: body mass index (BMI) ≥30kg/m2.
BP levels ≤130/80mmHg were considered adequate BP control in individuals with albuminuria (ACR>30mg/dL), BP levels ≤140/90mmHg were satisfactory for the remaining hypertensive patients.1
Information on pharmacological treatment was obtained from the pharmacy billing database, based on the ATC code of each active ingredient. The number of daily doses for each active ingredient taken from the pharmacy within ±6 months from the date of the baseline creatinine determination, with respect to the time interval between the first and last withdrawn was recorded. To estimate the level of exposure to each active ingredient, therapeutic compliance was defined as purchase of more than 60% of the total daily dose defined for the period. Administration of antihypertensive drugs affecting renal function (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitors, aldosterone antagonists and other diuretics) and other treatments that can modify cardiovascular risk (antiplatelet agents, lipid-lowering agents) was monitored.
Statistical analysisContinuous variables were described as median and interquartile range, while qualitative variables were expressed as frequency and percentage.
The Mann–Whitney U-test was used to compare quantitative variables and the chi-squared test for categorical variables between groups according to eGFR<60.
Multivariate logistic regression models adjusted for variables with p<0.10 in the bivariate analysis was used to determine the variables associated with eGFR<60 and the correlation of these with gender, age and DM. Any effects that detracted from the quality of the model were progressively eliminated according to the Akaike Information Criteria. The missing data were imputed according to the Markov Chain Monte Carlo Multiple Imputation method (5 imputations, 10 iterations; correlations included in the process). The resulting model was replicated without these data to observe the effect of data imputation.
Statistics were calculated using R, version 2.14.2 (R Foundation for Statistical Computing, Vienna, Austria).
The study was approved by the Independent Ethics Committee of the Jordi Gol Foundation for Primary Care Research (IDIAP).
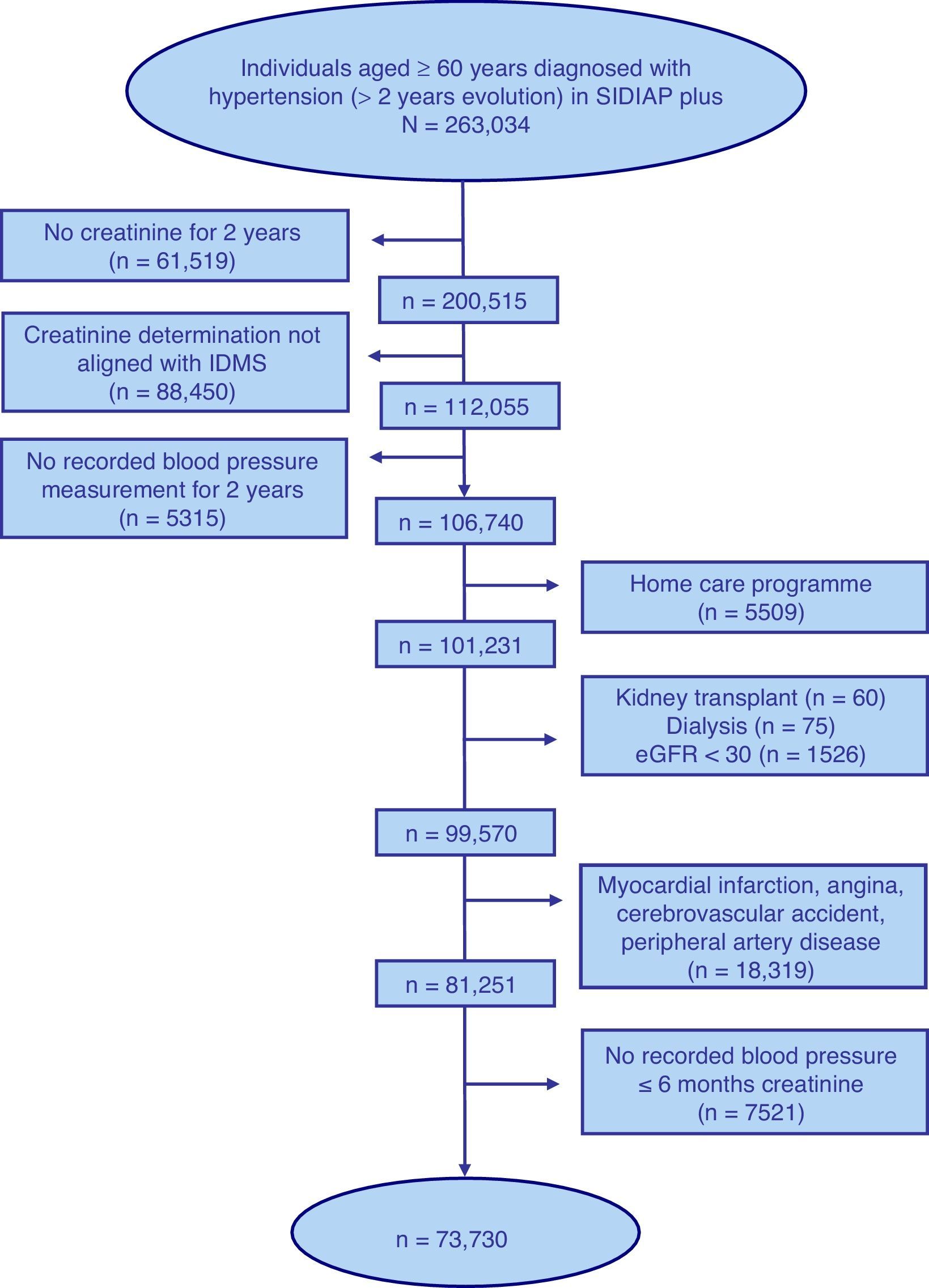
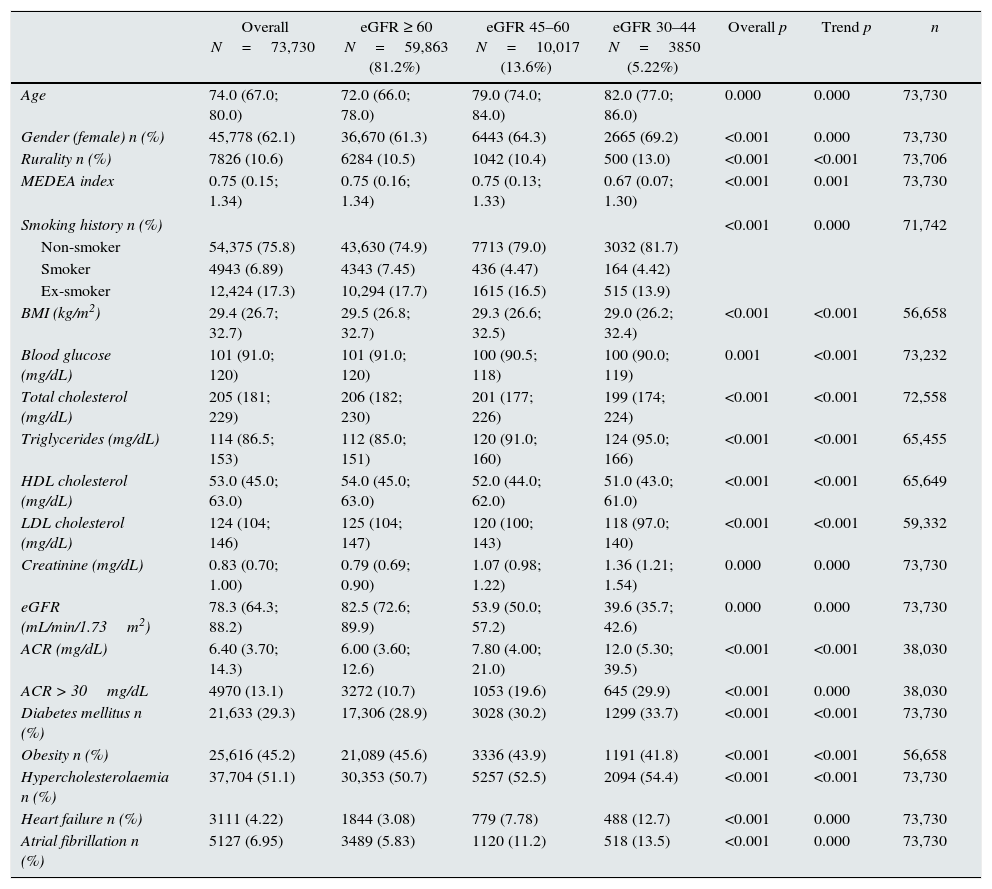
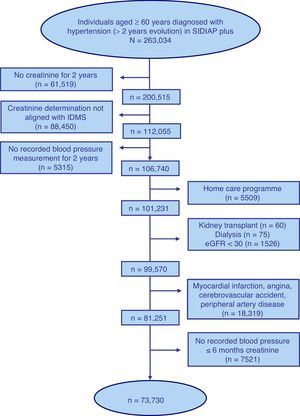
ResultsFig. 1 shows the selection process flow chart for the 263,034 individuals aged ≥60 years with a diagnosis of HTN of 2 or more years of follow up included in the SIDIAP plus database as of 01/01/2011. The final study population included 73,730 individuals with a median age of 74 years, of whom 62.1% were women (Table 1).
Selection process of the sample of individuals aged ≥60 years included in the SIDIAP plus database as of 1st January 2011, with a coded diagnosis of hypertension, a recent standardised serum creatinine determination and blood pressure measurement, and with no kidney failure, cardiovascular disease or home care.
Characteristics of the sample of hypertensive individuals aged ≥60 years, in the total sample selected, according to eGFRCKD-EPI ≥60, 45–59 or <30–44 (n=73,730), taken from the SIDIAP plus database as of 1 January 2011.
| Overall N=73,730 | eGFR ≥ 60 N=59,863 (81.2%) | eGFR 45–60 N=10,017 (13.6%) | eGFR 30–44 N=3850 (5.22%) | Overall p | Trend p | n | |
|---|---|---|---|---|---|---|---|
| Age | 74.0 (67.0; 80.0) | 72.0 (66.0; 78.0) | 79.0 (74.0; 84.0) | 82.0 (77.0; 86.0) | 0.000 | 0.000 | 73,730 |
| Gender (female) n (%) | 45,778 (62.1) | 36,670 (61.3) | 6443 (64.3) | 2665 (69.2) | <0.001 | 0.000 | 73,730 |
| Rurality n (%) | 7826 (10.6) | 6284 (10.5) | 1042 (10.4) | 500 (13.0) | <0.001 | <0.001 | 73,706 |
| MEDEA index | 0.75 (0.15; 1.34) | 0.75 (0.16; 1.34) | 0.75 (0.13; 1.33) | 0.67 (0.07; 1.30) | <0.001 | 0.001 | 73,730 |
| Smoking history n (%) | <0.001 | 0.000 | 71,742 | ||||
| Non-smoker | 54,375 (75.8) | 43,630 (74.9) | 7713 (79.0) | 3032 (81.7) | |||
| Smoker | 4943 (6.89) | 4343 (7.45) | 436 (4.47) | 164 (4.42) | |||
| Ex-smoker | 12,424 (17.3) | 10,294 (17.7) | 1615 (16.5) | 515 (13.9) | |||
| BMI (kg/m2) | 29.4 (26.7; 32.7) | 29.5 (26.8; 32.7) | 29.3 (26.6; 32.5) | 29.0 (26.2; 32.4) | <0.001 | <0.001 | 56,658 |
| Blood glucose (mg/dL) | 101 (91.0; 120) | 101 (91.0; 120) | 100 (90.5; 118) | 100 (90.0; 119) | 0.001 | <0.001 | 73,232 |
| Total cholesterol (mg/dL) | 205 (181; 229) | 206 (182; 230) | 201 (177; 226) | 199 (174; 224) | <0.001 | <0.001 | 72,558 |
| Triglycerides (mg/dL) | 114 (86.5; 153) | 112 (85.0; 151) | 120 (91.0; 160) | 124 (95.0; 166) | <0.001 | <0.001 | 65,455 |
| HDL cholesterol (mg/dL) | 53.0 (45.0; 63.0) | 54.0 (45.0; 63.0) | 52.0 (44.0; 62.0) | 51.0 (43.0; 61.0) | <0.001 | <0.001 | 65,649 |
| LDL cholesterol (mg/dL) | 124 (104; 146) | 125 (104; 147) | 120 (100; 143) | 118 (97.0; 140) | <0.001 | <0.001 | 59,332 |
| Creatinine (mg/dL) | 0.83 (0.70; 1.00) | 0.79 (0.69; 0.90) | 1.07 (0.98; 1.22) | 1.36 (1.21; 1.54) | 0.000 | 0.000 | 73,730 |
| eGFR (mL/min/1.73m2) | 78.3 (64.3; 88.2) | 82.5 (72.6; 89.9) | 53.9 (50.0; 57.2) | 39.6 (35.7; 42.6) | 0.000 | 0.000 | 73,730 |
| ACR (mg/dL) | 6.40 (3.70; 14.3) | 6.00 (3.60; 12.6) | 7.80 (4.00; 21.0) | 12.0 (5.30; 39.5) | <0.001 | <0.001 | 38,030 |
| ACR > 30mg/dL | 4970 (13.1) | 3272 (10.7) | 1053 (19.6) | 645 (29.9) | <0.001 | 0.000 | 38,030 |
| Diabetes mellitus n (%) | 21,633 (29.3) | 17,306 (28.9) | 3028 (30.2) | 1299 (33.7) | <0.001 | <0.001 | 73,730 |
| Obesity n (%) | 25,616 (45.2) | 21,089 (45.6) | 3336 (43.9) | 1191 (41.8) | <0.001 | <0.001 | 56,658 |
| Hypercholesterolaemia n (%) | 37,704 (51.1) | 30,353 (50.7) | 5257 (52.5) | 2094 (54.4) | <0.001 | <0.001 | 73,730 |
| Heart failure n (%) | 3111 (4.22) | 1844 (3.08) | 779 (7.78) | 488 (12.7) | <0.001 | 0.000 | 73,730 |
| Atrial fibrillation n (%) | 5127 (6.95) | 3489 (5.83) | 1120 (11.2) | 518 (13.5) | <0.001 | 0.000 | 73,730 |
ACR: urinary albumin/creatinine ratio; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Numerical variables are shown with the overall median [1st quartile; 3rdquartile] according to the eGFRCKD-EPI group, the p-value of the Kruskal–Wallis test of equal distribution of the variable according to the eGFRCKD-EPI group, and the p-value of the Spearman's rank correlation coefficient. Categorical variables are shown with the overall absolute frequency (relative frequency) according to the eGFRCKD-EPI group, the p-value of the chi-squared test of equal distribution of the variable according to the eGFRCKD-EPI group, and the p-value of the chi-squared linear trend test.
In total, 18.8% of the study subjects presented with moderate CKD; in 72.2% of these, eGFR was between 45 and 59mL/min. Prevalence was higher in women (19.9% in women versus 17.0% in men, p<0.05) and increased with age, from 5.88% at 60–69 years to 39.4% in subjects >80 years. Individuals with decreased eGFR were older, with a higher percentage of women, from rural areas, diabetes mellitus, dyslipidaemia, HF and atrial fibrillation, a lower percentage of smokers/ex-smokers and obesity, and a lower socioeconomic level (p<0.001), which increased significantly with the low eGFR level (global p and linear trend <0.001).
Urinary ACR was available in 51.6% of the individuals (50.9% in the eGFR≥60 group and 54.4% in the eGFR<60 group; p<0.001), it was pathological in 13.1%. Supplementary Table 1 shows the descriptive data with imputations.
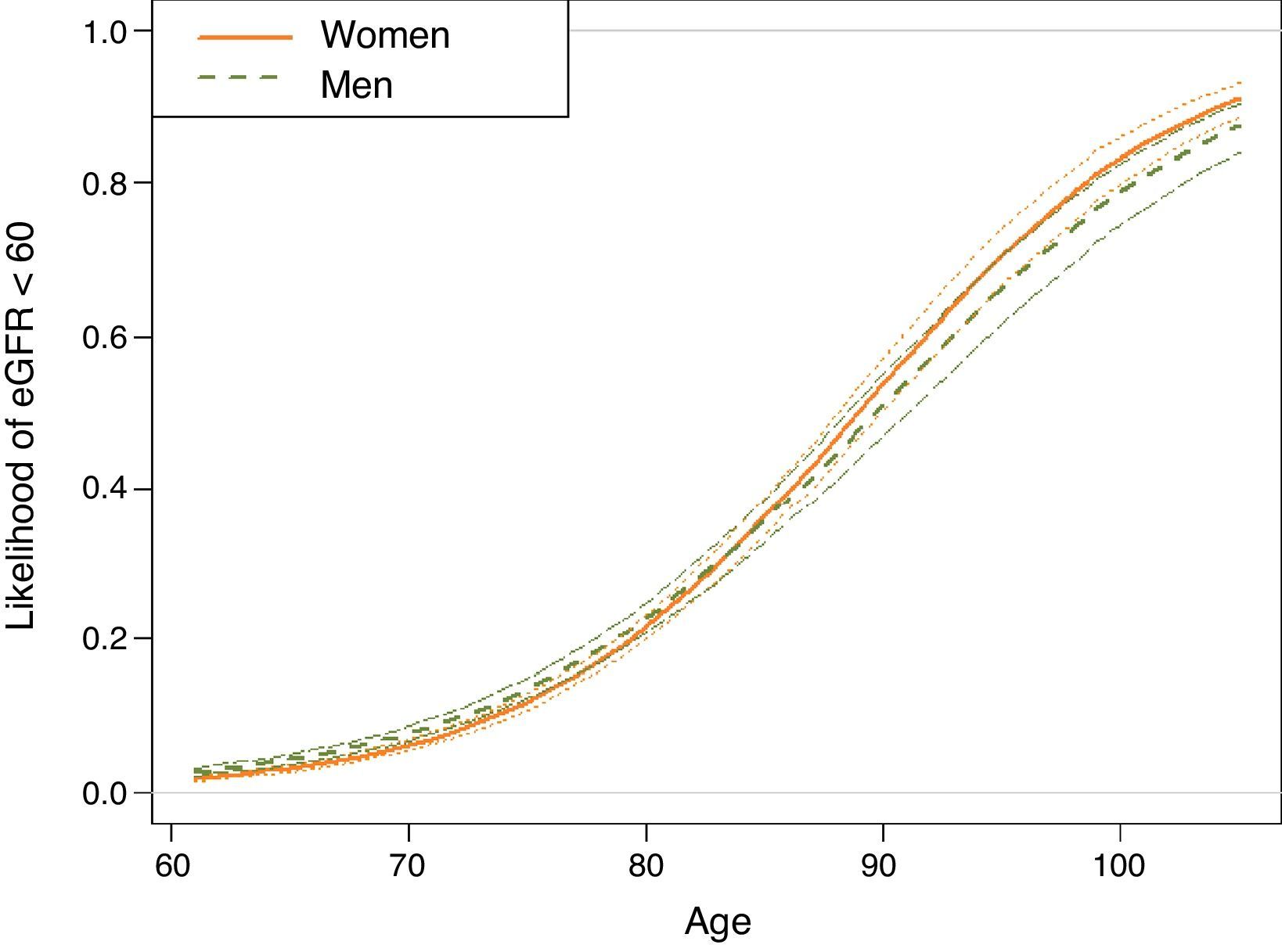
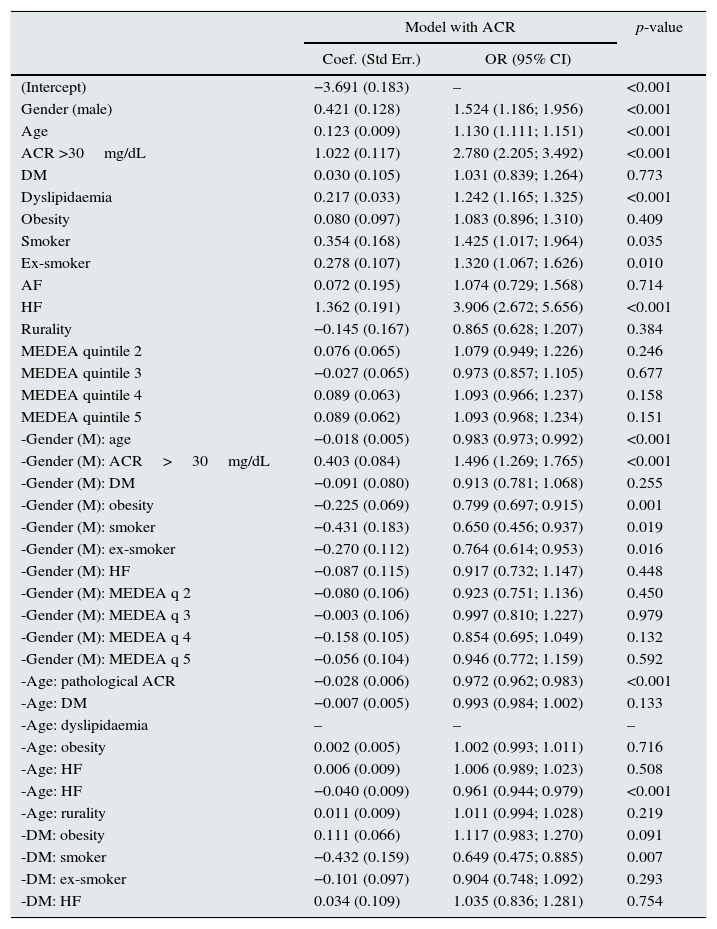
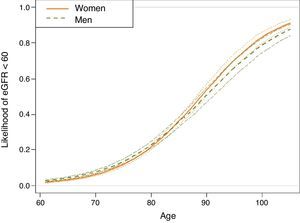
A multivariate analysis to assess the variables associated with eGFR<60 showed that gender, age, HF, ACR>30mg/dL, atrial fibrillation, smoking, dyslipidaemia, DM and obesity were significant factors (Table 2). Significant interactions were detected; the older the age of the patient, the weaker the correlation between male gender and eGFR<60 (Fig. 2). This correlation was inverted from the age of 80, when the risk of CKD was greater in women than in men. Correlation with age was similar for ACR>30mg/dL and diagnosis of HF and DM. In men, the correlation between decreased eGFR and pathological ACR increased, whereas the correlation between decreased eGFR and DM, obesity and smoking habit decreased. In the case of DM, the risk was greater in the presence of obesity and lower in smokers and ex-smokers.
Multivariate logistic regression model for eGFRCKD-EPI<60.
| Model with ACR | p-value | ||
|---|---|---|---|
| Coef. (Std Err.) | OR (95% CI) | ||
| (Intercept) | −3.691 (0.183) | – | <0.001 |
| Gender (male) | 0.421 (0.128) | 1.524 (1.186; 1.956) | <0.001 |
| Age | 0.123 (0.009) | 1.130 (1.111; 1.151) | <0.001 |
| ACR >30mg/dL | 1.022 (0.117) | 2.780 (2.205; 3.492) | <0.001 |
| DM | 0.030 (0.105) | 1.031 (0.839; 1.264) | 0.773 |
| Dyslipidaemia | 0.217 (0.033) | 1.242 (1.165; 1.325) | <0.001 |
| Obesity | 0.080 (0.097) | 1.083 (0.896; 1.310) | 0.409 |
| Smoker | 0.354 (0.168) | 1.425 (1.017; 1.964) | 0.035 |
| Ex-smoker | 0.278 (0.107) | 1.320 (1.067; 1.626) | 0.010 |
| AF | 0.072 (0.195) | 1.074 (0.729; 1.568) | 0.714 |
| HF | 1.362 (0.191) | 3.906 (2.672; 5.656) | <0.001 |
| Rurality | −0.145 (0.167) | 0.865 (0.628; 1.207) | 0.384 |
| MEDEA quintile 2 | 0.076 (0.065) | 1.079 (0.949; 1.226) | 0.246 |
| MEDEA quintile 3 | −0.027 (0.065) | 0.973 (0.857; 1.105) | 0.677 |
| MEDEA quintile 4 | 0.089 (0.063) | 1.093 (0.966; 1.237) | 0.158 |
| MEDEA quintile 5 | 0.089 (0.062) | 1.093 (0.968; 1.234) | 0.151 |
| -Gender (M): age | −0.018 (0.005) | 0.983 (0.973; 0.992) | <0.001 |
| -Gender (M): ACR>30mg/dL | 0.403 (0.084) | 1.496 (1.269; 1.765) | <0.001 |
| -Gender (M): DM | −0.091 (0.080) | 0.913 (0.781; 1.068) | 0.255 |
| -Gender (M): obesity | −0.225 (0.069) | 0.799 (0.697; 0.915) | 0.001 |
| -Gender (M): smoker | −0.431 (0.183) | 0.650 (0.456; 0.937) | 0.019 |
| -Gender (M): ex-smoker | −0.270 (0.112) | 0.764 (0.614; 0.953) | 0.016 |
| -Gender (M): HF | −0.087 (0.115) | 0.917 (0.732; 1.147) | 0.448 |
| -Gender (M): MEDEA q 2 | −0.080 (0.106) | 0.923 (0.751; 1.136) | 0.450 |
| -Gender (M): MEDEA q 3 | −0.003 (0.106) | 0.997 (0.810; 1.227) | 0.979 |
| -Gender (M): MEDEA q 4 | −0.158 (0.105) | 0.854 (0.695; 1.049) | 0.132 |
| -Gender (M): MEDEA q 5 | −0.056 (0.104) | 0.946 (0.772; 1.159) | 0.592 |
| -Age: pathological ACR | −0.028 (0.006) | 0.972 (0.962; 0.983) | <0.001 |
| -Age: DM | −0.007 (0.005) | 0.993 (0.984; 1.002) | 0.133 |
| -Age: dyslipidaemia | – | – | – |
| -Age: obesity | 0.002 (0.005) | 1.002 (0.993; 1.011) | 0.716 |
| -Age: HF | 0.006 (0.009) | 1.006 (0.989; 1.023) | 0.508 |
| -Age: HF | −0.040 (0.009) | 0.961 (0.944; 0.979) | <0.001 |
| -Age: rurality | 0.011 (0.009) | 1.011 (0.994; 1.028) | 0.219 |
| -DM: obesity | 0.111 (0.066) | 1.117 (0.983; 1.270) | 0.091 |
| -DM: smoker | −0.432 (0.159) | 0.649 (0.475; 0.885) | 0.007 |
| -DM: ex-smoker | −0.101 (0.097) | 0.904 (0.748; 1.092) | 0.293 |
| -DM: HF | 0.034 (0.109) | 1.035 (0.836; 1.281) | 0.754 |
Model based on gender, age of 60 years, ACR, DM, dyslipidaemia, obesity, smoking habit, HF, AF, rurality(from rural areas), MEDEA and the correlation between these variables and gender, age and DM (n=73,730); final model presented as a result of the selection of variables using the Akaike Information Criteria; imputation of missing data.
95% CI: 95% confidence interval of OR; ACR: urinary albumin–creatinine ratio; AF: atrial fibrillation; DM: diabetes mellitus; HF: heart failure; OR: odds ratio calculated as the exponential of the coefficients of the model; p: p-value of the coefficient of the logistic regression model.
The correlations presented in the table start with a “-” sign and a “:” between the 2 correlated effects.
Model adjusted for rurality and age:obesity, age:AF, age:rurality and DM:AF correlations.
Estimation, based on the logistic regression model, of the probability of subjects included in the sample of hypertensive individuals aged ≥60 years, taken from the SIDIAP plus database as of 1st January 2011, being in the glomerular filtration rate <60 group, according to age and gender (n=73,730).
Estimates for non-smokers with albumin/creatinine ratio <30mg/dL, without dyslipidaemia, obesity, atrial fibrillation, heart failure or diabetes, living in an urban environment and in the central quintile of the MEDEA socioeconomic level. For any other individual profile, the expected probabilities according to the logistic regression model differ from those shown. However, the effect of the age-gender correlation shown is maintained.
To observe the effect of imputations, the final multivariate model was replicated without them (Supplementary Table 2).
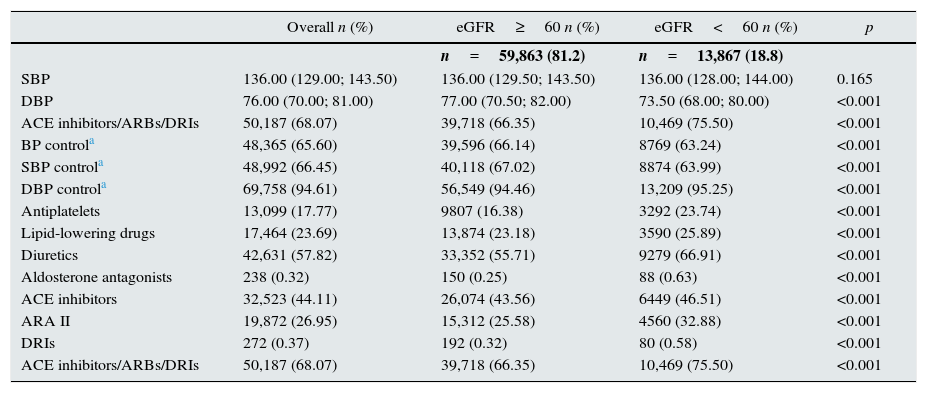
Blood pressure and exposure to drugsThe median SBP and DBP were 136 and 76mmHg, respectively (Table 3). SBP did not differ significantly among individuals with and without decreased eGFR, but DBP was lower in the group with kidney disease (p<0.001).
Blood pressure and exposure to drugs in the sample of hypertensive individuals aged ≥60 years, in the total sample selected, and according to eGFRCKD-EPI≥60 or ≥60 (n=73,730), taken from the SIDIAP plus database as of 1st January 2011.
| Overall n (%) | eGFR≥60 n (%) | eGFR<60 n (%) | p | |
|---|---|---|---|---|
| n=59,863 (81.2) | n=13,867 (18.8) | |||
| SBP | 136.00 (129.00; 143.50) | 136.00 (129.50; 143.50) | 136.00 (128.00; 144.00) | 0.165 |
| DBP | 76.00 (70.00; 81.00) | 77.00 (70.50; 82.00) | 73.50 (68.00; 80.00) | <0.001 |
| ACE inhibitors/ARBs/DRIs | 50,187 (68.07) | 39,718 (66.35) | 10,469 (75.50) | <0.001 |
| BP controla | 48,365 (65.60) | 39,596 (66.14) | 8769 (63.24) | <0.001 |
| SBP controla | 48,992 (66.45) | 40,118 (67.02) | 8874 (63.99) | <0.001 |
| DBP controla | 69,758 (94.61) | 56,549 (94.46) | 13,209 (95.25) | <0.001 |
| Antiplatelets | 13,099 (17.77) | 9807 (16.38) | 3292 (23.74) | <0.001 |
| Lipid-lowering drugs | 17,464 (23.69) | 13,874 (23.18) | 3590 (25.89) | <0.001 |
| Diuretics | 42,631 (57.82) | 33,352 (55.71) | 9279 (66.91) | <0.001 |
| Aldosterone antagonists | 238 (0.32) | 150 (0.25) | 88 (0.63) | <0.001 |
| ACE inhibitors | 32,523 (44.11) | 26,074 (43.56) | 6449 (46.51) | <0.001 |
| ARA II | 19,872 (26.95) | 15,312 (25.58) | 4560 (32.88) | <0.001 |
| DRIs | 272 (0.37) | 192 (0.32) | 80 (0.58) | <0.001 |
| ACE inhibitors/ARBs/DRIs | 50,187 (68.07) | 39,718 (66.35) | 10,469 (75.50) | <0.001 |
ACE inhibitors: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BP: blood pressure; DBP: diastolic blood pressure; DRIs: dopamine reuptake inhibitors; SBP: systolic blood pressure.
Numerical variables are shown with the overall median [1st quartile; 3rdquartile] and according to the eGFRCKD-EPI group, and the p-value of the Mann–Whitney U-test of equal distribution of the variable according to the eGFRCKD-EPI group. Categorical variables are shown with the overall absolute frequency (relative frequency), according to the eGFRCKD-EPI group, and the p-value of the chi-squared test of equal distribution of the variable according to the eGFRCKD-EPI group.
The level of BP control was 65.6% (66.5% in SBP and 94.6% in DBP). In individuals with decreased eGFR, BP control was lower overall and for SBP, and higher for DBP (p<0.05).
Exposure to antiplatelet, lipid-lowering and antihypertensive drugs was higher in hypertensive patients with CKD.
DiscussionThe prevalence of moderate CKD (eGFR 30–59mL/min/1.73m2) in ≥60 years individuals, with HTN of more than 2 years and without cardiovascular disease, was 18.8%. It was associated with older age, male gender, HF, pathological ACR, atrial fibrillation, smoking, dyslipidaemia, DM and obesity. Percent of BP control (overall 65.6%) was slightly less in individuals with decreased eGFR.
The prevalence of moderate CKD is comparable to that previously reported in hypertensive patients (17.2% in ≥55 years15 and 21.8% in primary care in Italy16) and lower than that reported in Spain (from 24.4% to 27.4%,17–19 and as high as 50% in hypertensive women aged ≥65 years20). These differences may be due to the use of non-standardised creatinine determinations and to the calculating eGFR using the MDRD study equation, which underestimates eGFR at higher values, especially in young individuals and women,9 as well as to the inclusion of individuals with previous cardiovascular disease associated with CKD.
This study shows a strong positive correlation between older age and increased risk of decreased eGFR. This association has been widely described both in the general population21 and in hypertensive individuals.15,16,18,19,22,23 In our study, age also affected other risk factors: the older the individual, the lesser the impact of male gender, pathological ACR, DM, atrial fibrillation and HF on CKD. This suggests that age predominates over the other risk factors in older individuals.
The correlation observed between gender and CKD is similar to that described in the literature. The prevalence of CKD is generally higher in women,15–18,22 but when adjusted for other risk factors, male gender is associated with the presence of CKD11,24 and a greater risk of progression to ESRD.25 The possibility that the eGFR equation is not correctly adjusted for age and gender cannot be ruled out. The CKD-EPI formula improves these aspects overall, compared with the MDRD study equation, but in the Three-city Prospective Cohort Study26 in older individuals, CKD-EPI did not show superiority in identifying individuals with poor prognosis.
In our analysis, pathological ACR was a risk factor for CKD, with more impact in men. Albuminuria is a known risk factor for CKD23,27 and progression to ESRD in hypertensive patients.5 In this respect, it is important to emphasise that ACR determination was available in only half of all study cases, and showed a minimum increase in eGFR<60, despite the recommendations of clinical guidelines for the management of HTN7 and CKD.1
In this study, decreased eGFR was also significantly correlated with HF and, to a lesser degree, with atrial fibrillation. The correlation with HF,24 left ventricular hypertrophy15,16,18 and cardiovascular disease15,16,18,20,22,28 has been described in other studies. HF is also a risk factor for CKD progression.29
The presence of other cardiovascular risk factors in hypertensive patients is known to increase the risk of CKD,19,20,27 and it is therefore considered a further manifestation of the atherosclerotic process. Smoking has been described as a predictor of albuminuria, especially in men, but not of eGFR<60.23,30 In our analysis, the increased risk of CKD associated with DM, obesity and smoking was lower in males. Interestingly, in the case of DM, the risk was greater in the presence of obesity and lower in smokers. All these effects may be assessed in greater detail in the follow-up of this cohort of hypertensive individuals aged ≥60 years.
There is some debate about ideal BP levels, which have varied in recent years and making comparisons difficult. The 65.6% BP control obtained in our population is comparable to the 61.1% with <140/90 described in hypertensive patients seen in Primary Care,31 while in individuals with CKD, 63.24% is comparable to the 60.2% described with the same criteria.32 These percentages are much higher than those published in previous studies,22,23 and are in line with the improvement observed in the control of HTN in recent years31 or in severe cases of HTN.20 However, this improvement should be interpreted with caution, since individuals with no BP data over the study period were not included in our analysis. It should be emphasised that individuals with CKD have worse BP control, despite greater exposure to drugs. This situation, which has also been described in other studies,16,22,24 shows the difficulty involved in controlling BP in these individuals. Nevertheless, some degree of therapeutic inertia or failure to prioritise BP control in these at-risk individuals cannot be ruled out.
The strength of this study is the large size of the population-based sample, which was taken from a validated and representative database used in cardiovascular epidemiology studies.13 Creatinine determination, which was carried out in several laboratories, was performed according to standardised methods aligned with IDMS.
Limitations of the study are its cross-sectional design, which prevents us from inferring causal associations. The results are representative of the hypertensive population without cardiovascular disease, treated and monitored in Primary Care. This population could possibly have a lower prevalence of decreased eGFR and better control of BP. The main objective of the project, of which this study is part, is to quantify the risk of cardiovascular events associated with a moderate decrease in eGFR. For this reason, individuals with previous cardiovascular disease were excluded, due to a higher incidence of new episodes and difficulties in distinguishing between new episodes and follow-up of previous ones using the data available in electronic medical records. BP records reflect standard clinical practice. As the specific circumstances of BP measurements are not recorded (single determination or the average of a series, rest conditions, etc.), the existence of heterogeneity among centres or professionals cannot be ruled out. The degree of BP control was not included in the multivariate analysis, because, in a cross-sectional study, it does not reflect the previous effect of BP on the presence of CKD. The analysis was performed using a single creatinine determination, which is common in epidemiological studies and also for ACR. Furthermore, data were not adjusted for race, because given the population characteristics in our setting, i.e., a clear predominance of Caucasians particularly in this age group, we believe this variable to be of little relevance. Finally, data for some variables were unavailable in many cases: regarding ACR, for example, as much as 48.8% of the data was missing. In these cases, the analysis of data without imputation could cause significant bias.
Despite its obvious advantages, multiple imputation of missing data using the Markov Chain Monte Carlo methods is not without its limitations. First, such method assumes that the missing data are random, that is, that they can be explained without bias by the remaining variables collected. Second, the imputation method incorporates a variability associated with the unavailability of the observed data, so it is less likely to detect associations with imputed data than if all data were observed. However, the final multivariate model replicated without imputations yielded very similar results, the main differences being a greater association between decreased eGFR and the rest of the risk factors when excluding ACR and the non-attenuation of the DM risk in men.
In conclusion, 1 out of 5 hypertensive individuals without cardiovascular disease, aged 60 or over and treated and monitored by Primary Care has a moderatedecreased eGFR, with albuminuria and HF as the main associated factors. Despite increased drug exposure, the control of BP control was poor in individuals with decreased eGFR. The follow-up of this cohort with and without moderately decreased eGFR will allow us to identify the factors associated with greater progression of kidney failure and incidence of cardiovascular events in our setting. Given the relevance of albuminuria in both the progression of CKD and cardiovascular risk, we believe that strategies to increase this determination in Primary Care should be explored, particularly in patients with kidney disease, and BP control should be prioritised and optimised in these individuals.
CommentsThis study was carried out with anonymised data from Primary Care electronic medical records provided by SIDIAP (Information System for the Development of Research in Primary Care). To use these data, the project was evaluated by the Scientific Committee of SIDIAP and approved by the Independent Ethics Committee of the Jordi Gol Foundation for Primary Care Research (IDIAP).
FundingThis study was supported by a grant from SIDIAP (Information System for the Development of Research in Primary Care).
Conflicts of interestThe authors declare that they have no conflicts of interest.
M. Jesús Cerain Herrero, Esther Freixes Villaró, Neus Gil Terrón, Mercedes Rodríguez Pascual, Laura Ruipérez Guijarro, Lluïsa Rodríguez Latre, Isabel Rosich Martí, Gemma Rodríguez Palomar, Jesús Almeda Ortega, Francisco Javier Tovillas Morán and Alberto Martínez Castelao.
Please cite this article as: Salvador-González B, Mestre-Ferrer J, Soler-Vila M, Pascual-Benito L, Alonso-Bes E, Cunillera-Puértolas O, et al. Enfermedad renal crónica en individuos hipertensos ≥60 años atendidos en Atención Primaria. Nefrologia. 2017;37:406–414.
MACAP Renal. Research group recognised by the Generalitat of Catalonia (i.e., the local government of Catalonia) (SGR 2014-2016).
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access