Introducción: La normohidratación es uno de los mayores objetivos en hemodiálisis (HD) y diálisis peritoneal (DP). La bioimpedancia por espectroscopia (BIS) se postula como el método más prometedor para la evaluación y seguimiento del estado de hidratación en pacientes en diálisis. Objetivo: Comparar la composición corporal de pacientes prevalentes en HD y DP en un intervalo de seis meses. Pacientes y métodos: Estudio observacional de 62 pacientes en HD y 19 en DP comparando los parámetros clínicos, bioquímicos y de bioimpedancia. Resultados: En el estudio comparativo, los pacientes en DP fueron más jóvenes (50 ± 10 vs. 57 ± 14 años, p = 0,031). El índice de comorbilidad de Charlson (4,8 ± 3 vs. 7,5 ± 3, p < 0,001), tiempo en diálisis (16,9 ± 18,01 vs. 51,88 ± 68,79 meses, p = 0,020) y proteína C reactiva [3 (3-9,3) vs. 5,25 (1-76,4)] fueron menores. Los niveles de proteínas totales (7,46 ± 0,44 vs. 7,04 ± 0,55 g/dl, p = 0,005), y transferrina (205 ± 41 vs. 185 ± 29 mg/dl, p = 0,024) fueron más elevados. BIS: agua intracelular (AIC) (19,67 ± 3,61 vs. 16,51 ± 3,36 litros, p = 0,010), masa muscular total (MM) (37,20 ± 8,65 vs. 32,57 ± 8,72 kg, p = 0,029), masa celular total (MCT) (20,53 ± 5,65 vs. 17,56 ± 5,91 kg, p = 0,033) y ángulo de fase (Phi 50) (5,81 ± 0,86 vs. 4,74 ± 0,98, p = 0,000) fueron más elevados que en HD. Sobrehidratados 22% en HD y 10% en DP, en las condiciones referidas en métodos. A los seis meses en DP observamos aumento de peso (73,75 ± 12,27 vs. 75,22 ± 11,87 kg, p = 0,027), grasa total (MG) (26,88 ± 10 vs. 30,02 ± 10 kg, p = 0,011) y relativa (MG %) (35,75 ± 9,87 vs. 39,34 ± 9,12, p = 0,010); disminución de AIC (18,56 ± 3,45 vs. 17,65 ± 3,69 l, p = 0,009), MM (36,95 ± 8,88 vs. 34 ± 9,70 kg, p = 0,008) y MM relativa (MM %) (50,85 ± 12,33 vs. 45,40 ± 11,95%, p = 0,012). En el análisis multivariante, la variación (¿) de peso guarda relación con el ¿ de grasa (p < 0,001). Encontramos correlación entre el incremento de grasa y el decremento de masa muscular (p = 0,01). A los seis meses en HD no se observaron cambios en estos parámetros, salvo una reducción en el agua extracelular (15,11± 2,45 vs. 14,00 ± 2,45, p = 0,001). Conclusiones: BIS permite valorar los cambios en la composición corporal y ayuda a establecer el peso seco e introducir cambios en las pautas de tratamiento.
Introduction: Proper hydration is one of the major aims in haemodialysis (HD) and peritoneal dialysis (PD). Bioimpedance spectroscopy appears to be a promising method for the evaluation and follow up of the hydration status in dialysis patients (P). Objectives: We compared body composition between stable patients on HD and PD after six months. Patients and method: An observational study with 62 P on HD and 19 P on PD was performed. Clinical, biochemical and bioimpedance parameters were analysed. Results: In the comparative study, PD P were younger (50±10 vs 57±14 years, P=.031). The Charlson Comorbidity Index (4.8±3 vs 7.5±3, P<.001), time on dialysis (16.9±18.01 vs 51.88±68.79 months, P=.020) and C-Reactive Protein [3 (3-9.3) vs 5.25 (1-76.4)] were lower. Total protein levels (7.46±0.44 vs 7.04±0.55 g/dl, P=.005) and transferrin levels (205±41 vs 185±29 mg/dl, P=.024) were higher. BIS: Intracellular water (19.67±3.61 vs 16.51±3.36 litres, P=.010), lean tissue mass (LTM) (37.20±8.65 vs 32.57±8.72 kg, P=.029), total cellular mass (TCM) (20.53±5.65 vs 17.56±5.91 kg, P=.033), and bioelectrical impedance phase angle (Phi 50) (5.81±0.86 vs 4.74±0.98, P=.000) were higher than in HD P. Overhydration: 22% in HD y 10% in PD, in conditions referred to in methods. Six months later, PD P increased in weight (73.75±12.27 vs 75.22±11.87 kg, P=.027), total fat (FAT) (26.88±10 vs 30.02±10 kg, P=.011) and relative fat (Rel FAT) (35.75±9.87 vs 39.34±9.12, P=.010); and decreased in ICW (18.56±3.45 vs 17.65±3.69 l, P=.009), LTM (36.95±8.88 vs 34±9.70 kg, P=.008) and relative LTM (Rel LTM) (50.85±12.33 vs 45.40±11.95%, P=.012). In the multivariate analysis, weight variation (¿) was related to ¿ FAT (P<.001). We found a correlation between fat increase and lean tissue mass decrease. Six months later, in HD P, we observed a reduction in ECW (15.11±2.45 vs 14.00±2.45, P.001), without changes in other parameters. Conclusions: Bioelectrical impedance analysis facilitates the assessment of changes in body composition so as to correct dry weight and to introduce changes in treatment schedule.
INTRODUCTION
Achieving a normal hydration state is one of the primary objectives in haemodialysis (HD) and peritoneal dialysis (PD) treatments. The concept of dry weight is essential to integrated dialysis therapy.1 The abnormal state of overhydration has been related to arterial hypertension, signs and symptoms of pulmonary and peripheral oedema, heart failure, left ventricular hypertrophy, and other adverse cardiovascular effects.2 The increase in left ventricular mass is correlated with worse cardiovascular evolution in PD patients,3 and it has also been described that hydration state is an important independent predictor for mortality in chronic HD patients.4 It appears essential that dialysis providers have a good strategy for maintaining the euvolemic state of their patients. However, the evaluation of normovolemia is difficult, since there is no method that has been established for use in daily clinical practice. The clinical evaluation of dry weight is the most commonly used method, but this leads to frequent conditions of sub-clinical over-hydration and sub-hydration, which can cause increased morbidity rates.5 Among the various tests that can be used to measure dry weight, chest x-ray aids in the clinical management of patients, but does not comply with the aims of being rapid and non-invasive; the diameter of the inferior vena cava and its respiratory variations are good measures of preload,6 but they are also influenced by cardiovascular factors such as diastolic dysfunction, pulmonary hypertension, and chronic obstructive pulmonary disease.7 Biochemical markers such as ANP (atrial natriuretic peptide) have a prognostic value and may indirectly reflect overhydration due to its effect on left ventricular mass,5 but these levels appear to depend more on the primary situation of the ventricle. It is difficult to establish the proper concentration in dialysis patients, and values frequently remain elevated in patients considered to be properly hydrated.8
The standard methods for measuring body water such as deuterium and sodium bromide for extracellular water are laborious and are not commonly used in clinical practice.9
The new bioelectrical impedance analysis techniques are being used for the evaluation and follow-up of hydration state. In a study evaluating the detection limits for different methods used for determining hydration states in dialysis patients, bioimpedance spectroscopy has demonstrated high sensitivity, emerging as the most promising method for a practical treatment of dialysis patients.7 This technique uses the variation in the electrical frequency applied and distinguishes between extracellular water and total body water. The variation in frequency applying currents that range between 5kHz and 1000kHz facilitates the determination of extracellular water (ECW) and total body water (TBW); intracellular water (ICW) is extrapolated by analysing at different frequencies.8,10
It has been well established that the changes produced over time in body weight among peritoneal dialysis patients are due to changes both in body water content and lean tissue/fat mass. Multifrequency bioelectrical impedance analysis offers the possibility of evaluating body composition and hydration state.11
The body composition monitor (BCM, Fresenius Medical Care) has been validated for use in clinical practice to determine hydration state.10 As measured by BCM, a relative overhydration greater than 15% has been shown to be associated with increased cardiovascular mortality in haemodialysis patients.4
Recently, overhydration has also been correlated with inflammation, malnutrition, and atherosclerosis in PD patients.12
The objective of our study is to compare the body composition of prevalent HD and PD patients in a cross-sectional analysis, and to evaluate the changes in these values by performing two studies 6 months apart in both techniques, HD and PD, in a dialysis unit with special attention to maintaining dry weight.
METHODS
Patients
We performed a cross-sectional study of prevalent patients on HD (n=62) and PD (n=19) monitored at the same centre for a period that included two measurements of bioelectrical impedance with an interval of 6 months. The number of patients at the start of the study was 65 on HD and 19 on PD. After 6 months, there were 49 on HD and 14 on PD. All patients were older than 18 years. We excluded those patients with contraindications for bioelectrical impedance: an implanted electronic device, any type of metallic implants, amputation, pregnancy, and lactating women. All patients signed an informed consent that was approved by the ethics committee.
Of the 62 patients on HD, 21 used online haemodiafiltration and 41 were on conventional HD. Of the 19 patients on PD, 10 were on automated PD (APD) and 9 were on continuous ambulatory PD (CAPD); icodextrin was administered in 49% of patients on both techniques, and 23 patients (27%) were diabetic.
Measurements
In the initial cross-sectional study, we compared the following clinical parameters for the two techniques: age, sex, Charlson comorbidity index,13 time on dialysis, weight, body mass index (BMI), and systolic and diastolic blood pressure (BP).
Laboratory analyses: we measured C-reactive protein (CRP) using immunoturbidimetry, and creatinine, total protein, albumin, transferrin, and haemoglobin were measured using certified methods in the biochemistry department of the Severo Ochoa Hospital. The erythropoietin resistance index was defined as the weekly doses of erythropoietin (U/kg predialysis/dose) divided by haemoglobin (Hb) g/dl.
We performed the predialysis bioelectrical impedance analysis immediately before the second session of the week, and in PD patients, coinciding with the peritoneal equilibration test and with the peritoneum full. We used a body mass composition analysis device using bioimpedance spectroscopy (BCM, Fresenius Medical Care). The parameters for bioelectrical impedance were: TBW in litres (l), ECW in l, ICW in l, ECW/ICW, lean tissue mass (LTM) in kg, LTM% in percentage, lean tissue mass index in kg/m2, total fat (FAT) in kg, FAT% in percentage, phase angle (Phi 50), total cellular mass (TCM) in kg, and overhydration (OH) in l. The state of OH was calculated by standardising OH to ECW and considering OH to be present if >15%.4
The clinical, biochemical, and bioelectrical impedance values were analysed after six months for both dialysis techniques.
Statistical Analysis
We performed all statistical analyses using SPSS statistical software version 12 (Chicago, Illinois, SL, USA). Normally distributed variables were expressed as a mean and standard deviation, and non-normal variables as a median and range (maximum and minimum). We compared the means between groups using Student’s t-tests or Mann-Whitney U-tests and/or chi-square tests according to the nature of the variable. Categorical variables were expressed as number and percentage. We set the value of statistical significance at P<.05. For the univariate analysis, we used Pearson’s or Spearman’s correlation coefficients, according to the nature of the variable. The multivariate analysis involved linear correlation, considering the increase in weight as the dependent variable, and individually introducing variables that were statistically significant in the univariate analysis.
RESULTS
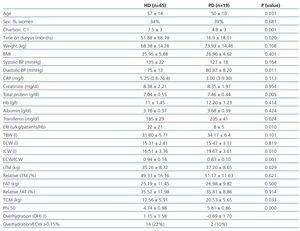
We analysed the data from a total of 65 patients on HD and 19 on PD. The demographic, clinical, biochemical, and bioelectrical impedance values from the initial analysis are summarised in Table 1. The patients on PD were younger (50±10 years vs 57±14 years, P=.031), with a lower Charlson comorbidity index (4,8±3 vs 7,5±3, P<.001), less time on dialysis (16.9±18.01 months vs 51.88±68.79 months, P=.020), lower CRP [3 (3-9.3) vs 5.25 (1-76.4)] and had higher values of total protein (TP) (7.46±0.44g/dl vs 7.04±0.55g/dl, P=.005) and transferrin (205±41mg/dl vs 185±29mg/dl, P=.024) than HD patients. Patients on PD had a residual renal function (RRF) of 5.33±3.89ml/min, diuresis at 1115±758ml/day, and ultrafiltration at 887±445ml/day. The ultrafiltration volume per session of HD patients on the day of bioelectrical impedance analysis was 2372±921ml.
As regards the bioelectrical impedance analysis parameters, PD patients had values of ICW (19.67±3.61 vs 16.51±3.36 litres, P=.010), LTM (37.20±8.65kg vs 32.57±8.72kg, P=.029), TCM (20.53±5.65kg vs 17.56±5.91kg, P=.033) and phase angle (5.81±0.86 vs 4.74±0.98, P=.000) greater than HD patients. Under the conditions the study was being carried out in, a total of 14 HD patients and 2 PD patients were overhydrated (22% and 10%, respectively).
Diabetic patients had a higher mean systolic BP (P=.012) and lower phase angle (P=.008), with no difference in the rest of the parameters used to measure body composition.
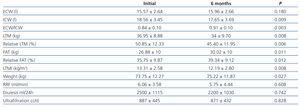
In patients on PD after 6 months (table 2), we observed a significant increase in weight (73.75±12.27kg vs 75.22±11.87kg, P=.027), with an increase in total fat (26.88±10kg vs 30.02±10kg, P=.011) and relative fat (35.75±9.87% vs 39.34±9.12%, P=.010), and decreased ICW (18.56±3.45l vs 17.65±3.69l, P=.009), total LTM (36.95±8.88kg vs 34±9.70kg, P=.008) and relative LTM (50.85±12.33% vs 45.40±11.95%, P=.012). There was a global correlation between the variation (¿) in weight and ¿ in fat, but not with ¿ in extracellular weight. In the multivariate analysis, the ¿ in weight was correlated with ¿ in fat (P<.001). There was also a correlation between the increase in fat and a decrease in LTM (P=.01). In the multivariate analysis that held the decrease in LTM as the dependent variable and a progressive introduction of age, sex, dialysis technique, and BMI, only age had an influence on the decrease in LTM (P=.012).
Upon analysing the data according to the technique of PD used, we observed a tendency towards lower increase in fat when on APD (1.58±3.05 vs 3.5±3.05), despite a higher glucose load (206±58 vs 62.98±75.5, P=.006).
In patients on HD, only ECW was significantly reduced (P=.001); all other parameters measured using bioelectrical impedance did not vary in the measurements taken over the 6-month interval (Table 3).
DISCUSSION
This study demonstrates the differences in body composition between patients on HD and those on PD. In the initial study, the differences in the nutritional parameters evaluated (TP, transferrin) and bioelectrical impedance (ICW, LTM, TCM, and phase angle) can be attributed to the younger age, less time on dialysis, and better nutritional state in the group on PD. Despite this, a significant weight gain is evident over the six-month observation period among patients on PD, which is not produced in patients on HD. The weight gain is primarily in the form of fat mass. These data could indicate on the one hand that the glucose input from PD could be responsible for the fat increase in these patients, and that it is more difficult to control extracellular volume in PD than in HD, as a consequence of the progressive reduction in RRF.
Patients on HD experienced a decrease in ECW with no variations in the other parameters measured over the six-month period.
LTM decreases in patients on PD, probably secondary to the more sedentary lifestyle associated to dialysis, which could contribute to the increase in fat content in PD patients, and also probably indicates the need for a physical exercise regimen. We were surprised that the decrease in LTM was not significant in HD patients, which we believe could be due to the short time span between the two measurements. We will continue with more prolonged follow-up times.
In our study, 22% of the HD patients and 10% of the PD patients were overhydrated, according to the criteria established by other publications.4 We did not perform post-dialysis bioelectrical impedance analysis, since it requires at least 30 minutes to carry out the procedure and the patients would not consent. Even so, in the case of HD, we evaluated the level of overhydration in the patients’ maximum state of overhydration (pre-HD), and we doubt that the two situations would be comparable.
When pre and post-HD measurements were available, and overhydration was calculated over the mean at centres that took similar care to reach dry weight in each dialysis session, 10% of patients were overhydrated,11 similar to our patients on PD.
Our results indicate that our patients on PD gain weight above all due to fat increase. Additionally, a slight increase in ECW, probably due to a slight decrease in diuresis, contributes to the weight gain observed, although these changes were not statistically significant.
In our patients on PD, the prevalence of overhydration (10%) was lower than reported from other centres.15,16
As has been shown, BP control is harder on automated PD than CAPD due to a worse negative sodium balance on APD resulting from a sodium sieving coefficient in the peritoneal membrane.17,18 The use of icodextrin can favour improved control of the volume situation and reduce left ventricular mass.19 In our experience,20 there is no difference in controlling BP or residual renal function between the two types of PD, probably due to the insistence in restricting salt from the diet and ample use of icodextrin.
The tendency in our patients on APD was for a lower increase in fat, despite a greater glucose load than in CAPD, perhaps due to the reduced time the glucose spends in the peritoneal cavity and thus lower total absorption.
The high prevalence of arterial hypertension and volume overload in HD centres and the difficulty for establishing dry weight in dialysis patients21 situates bioelectrical impedance as another tool for evaluating the changes suffered in body composition that can orient the physician to establish dry weight in HD patients and to introduce changes in the liquids provided to PD patients.
Table 1. Clinical, biochemical, and bioelectrical impedance parameters from the initial analysis
Table 2. Evolution after 6 months in the 14 patients on peritoneal dialysis
Table 3. Evolution after 6 months in the 49 patients on haemodialysis