Patients with chronic kidney disease (CKD) are susceptible to SARS-CoV-2 infection and more prone to develop severe disease. It is important to know predictors of poor outcomes to optimize the strategies of care.
Methods93 patients with CKD and 93 age-sex matched patients without CKD were included in the study. Data on demographic, clinical features, hematological indices and outcomes were noted and compared between the groups. Neutrophile to lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII) (platelet counts×neutrophil counts/lymphocyte counts) and lymphocyte-to-CRP ratio (LCR) were calculated on admission and the association of these markers with disease mortality in CKD patients was identified.
ResultsCKD patients had higher risk of severe disease, and mortality compared to non-CKD patients (72% vs 50.5%, p=0.003, 36.6% vs 10.8%, p<0.001, respectively) and were more likely to have higher values of immuno-inflammatory indices (leukocyte count, neutrophil, NLR, SII and C-reactive protein, etc.) and lower level of lymphocyte and LCR. Also, higher levels of NLR, SII, PLR and lower level of LCR were seen in CKD patients who died compared to those recovered. In a receiver operating characteristic curve analysis, NLR, SII, PLR and LCR area under the curve for in-hospital mortality of CKD patients were 0.830, 0.811, 0.664 and 0.712, respectively. Among all parameters, NLR and SII gave us the best ability to distinguish patients with higher risk of death. Based on the cut-off value of 1180.5, the sensitivity and specificity of the SII for predicting in-hospital mortality were found to be 67.5% and 79.6%, respectively. The corresponding sensitivity and specificity of the NLR were 85.2% and 66.1%, respectively, at the cut-off value of 5.1. Forward stepwise logistic regression analysis showed that NLR (≥5.1), SII (≥1180.5) and LCR (≤9) were predictors for in-hospital mortality.
ConclusionWe report for the first time that SII is able to distinguish COVID-19 infected CKD patients of worse survival and it is as powerful as NLR in this regard. As SII is easily quantified from blood sample data, it may assist for early identification and timely management of CKD patients with worse survival.
Los pacientes con enfermedad renal crónica (ERC) son susceptibles a la infección por SARS-CoV-2 y más propensos a desarrollar una enfermedad grave. Es importante conocer los predictores de los malos resultados para optimizar las estrategias de atención.
MétodosSe incluyeron en el estudio 93 pacientes con ERC y 93 pacientes sin ERC, emparejados por edad y sexo. Los datos sobre las características demográficas, clínicas, índices hematológicos y resultados, se anotaron y compararon entre los grupos. La proporción de neutrófilos a linfocitos (NLR), la proporción de plaquetas a linfocitos (PLR), el índice de inflamación inmunitaria sistémica (SII) (recuentos de plaquetas×recuentos de neutrófilos/recuentos de linfocitos) y la proporción de linfocitos a PCR (LCR) se calcularon en el momento de la admisión y se identificó la asociación de estos marcadores con la mortalidad por enfermedad en pacientes con ERC.
ResultadosLos pacientes con ERC tuvieron un mayor riesgo de enfermedad grave y mortalidad en comparación con los pacientes sin ERC (72% vs 50,5%, p=0,003, 36,6% vs 10,8%, p < 0,001, respectivamente) y tuvieron más probabilidades de tener valores más altos de índices inmuno inflamatorios (recuento de leucocitos, neutrófilos, NLR, SII y proteína C reactiva, etc.) y niveles más bajos de linfocitos y LCR. Además, se observaron niveles más altos de NLR, SII, PLR y un nivel más bajo de LCR en pacientes con ERC que murieron en comparación con los recuperados. En un análisis de la curva de características operativas del receptor, el área NLR, SII, PLR y LCR bajo la curva de mortalidad hospitalaria de pacientes con ERC fueron de 0,830, 0,811, 0,664 y 0,712, respectivamente. Entre todos los parámetros, NLR y SII se dió a conocer la mejor manera de distinguir a los pacientes con mayor riesgo de muerte. Con base en el valor de corte de 1180,5, se encontró que la sensibilidad y especificidad del SII, para predecir la mortalidad hospitalaria, fue del 67,5% y 79,6%, respectivamente. La sensibilidad y especificidad correspondientes del NLR fueron del 85,2% y 66,1%, respectivamente, en el valor de corte de 5,1.
El análisis de regresión logística escalonada hacia adelante mostró que el NLR (≥5,1), SII (≥1180,5) y LCR (≤9) fueron predictores de mortalidad hospitalaria.
ConclusiónInformamos, por primera vez, que el SII es capaz de distinguir pacientes con ERC infectados por COVID-19 de peor supervivencia y, en este sentido, es tan poderoso como el NLR. Como el SII se cuantifica fácilmente a partir de los datos de las muestras de sangre, puede ayudar a la identificación temprana y el manejo oportuno de los pacientes con ERC con peor supervivencia.
Since the outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, more than 162 million cases and 3.3 million deaths have been reported as of May 19, 2021 in the world.1 Also, with 5,117,374 confirmed cases and 44,760 deaths, Turkey is one of the most affected countries during the COVID-19 pandemic.2 The clinical manifestations of COVID-19 include fever, cough, fatigue, muscle aches, diarrhea, and pneumonia, which can develop into acute respiratory distress syndrome (ARDS), metabolic acidosis, and even liver, kidney or heart failure.3 Comorbidities such as hypertension (HT), diabetes mellitus (DM), coronary heart disease (CHD), cerebrovascular disease, chronic obstructive pulmonary disease, and kidney disorders are risk factor for disease severity and fatality.4 The risk for COVID-19 death in patients with chronic kidney disease (CKD) is greater than the risk for COVID-19 death in patients with DM and CHD and the risk increases as the eGFR decreases, with the highest risk in patients on renal replacement therapy.5 Increased risk of infectious complications and more adverse outcomes in CKD patients can be attributed to older age, additional comorbidities, pro-inflammatory state and the alterations of the innate and adaptive immune response associated with uremia.6–9 Therefore, early detection and accurate evaluation of the severity of SARS-CoV-2 infection in CKD patients may facilitate appropriate clinical decision making.
Although, primarily it was documented as a respiratory tract infection, COVID-19 is a systemic disease with a significant impact on the hematopoietic and immune system. The alterations in circulating blood cells related with inflammation and immune status of COVID-19 positive patients have been reported.10 Hematological parameters, such as white blood cells and their subpopulations, red cell distribution width, mean platelet volume, and platelet, and combined ratios of these parameters such as neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) are widely used for risk stratification, diagnosis, and determination of prognosis.10,11 Systemic immune inflammation index (SII) based on peripheral lymphocyte, neutrophil and platelet counts has been considered as a better index to reflect the local immune response and systemic inflammation. Recently, it was reported that the elevated SII was a prognostic indicator in predicting in-hospital mortality of COVID 19.12
To date, few studies have assessed the prognostic capacity of blood cell count derived inflammation indexes in CKD patients. Therefore, we aimed to investigate changes in hematological parameters and indexes in CKD patients with SARS-CoV-2 infection, in comparison with patients without CKD and to evaluate their utility as prognostic markers of disease mortality in CKD patients. To the best of our knowledge, this is the first time that these markers have been investigated simultaneously in a single study conducted on CKD patients.
Materials and methodsStudy design and participantsThis was a retrospective study performed on COVID-19 patients with CKD, including moderate and advanced CKD patients (stage 3–5 CKD) and maintenance hemodialysis (HD) patients. CKD patients matched one to one to age and sex matched COVID-19 patients without biochemical and/or radiological evidence of kidney disease. All included patients were symptomatic and had either a positive result in real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of nasal and pharyngeal swab samples or chest computerized tomography findings compatible with COVID 19. Exclusion criteria were acute kidney injury at admission, presence of hematological malignancies and concurrent chemotherapy or immunosuppressive treatment.
Data collection and definitionsData were obtained from electronic medical records, including demographics, co-morbid diseases, clinical features, laboratory findings at admission, length of hospitalization, and outcomes. Severity score of chest computerized tomography (CT) proposed by Pan et al. were also recorded.13 Routine laboratory examination consisted of complete blood count analysis including hemoglobin, leucocytes, platelets, absolute neutrophil and lymphocyte counts as well as serum biochemical tests (including renal and liver function, lactate dehydrogenase), D-dimer, fibrinogen, ferritin, C-reactive protein (CRP) and procalcitonin (PCT). Blood cell count derived inflammation indexes; NLR (Neutrophil count/Lymphocyte count×100%), PLR (Platelet count/Lymphocyte count×100%), SII (platelet counts×neutrophil counts/lymphocyte counts), and lymphocyte-to-CRP ratio (LCR) (lymphocyte count/CRP value) were calculated.
Severe COVID-19 was defined as patients that met any of following criteria: respiratory frequency more than 30/minute, oxygen saturation under 92% and/or the partial pressure of arterial oxygen and the inspiratory oxygen fraction (PaO2/FiO2) ratio less than 300. Intensive care need of those with severe disease were noted.
The primary endpoint was all-cause mortality. We assessed in-hospital mortality defined by survival status at discharge. All parameters and outcomes were compared between patients with CKD and without CKD. Also, blood cell derived inflammation indexes were compared between CKD patients who died and recovered.
Approval from the local ethics committee was obtained for this study (confirmation date and number: February 15, 2020/2021-04-10). This study was conducted in accordance with the principles of the Declaration of Helsinki.
StatisticsStatistical analyses were performed by NCSS (Number Cruncher Statistical System) with statistical significance set at two-tailed p<0.05. Categorical variables were described as the total number and percentages and continuous variables were described as median interquartile range (IQR). A Kolmogorov–Smirnov test and Shapiro–Wilk test were used to evaluate the distribution of the sample data. Qualitative data were compared by using Pearson Chi-Square test and Fisher–Freeman–Halton Exact test, as appropriate. Mann–Whitney U test was used comparison of data that were not compatible with normal distribution. A receiver operating characteristic (ROC) curve analysis was adopted to determine the optimal cut-off point for NLR, PLR, LCR and SII with respect to survival. Forward logistic regression analysis was performed to identify variables associated with in-hospital mortality in terms of odds ratio and 95% confidence intervals.
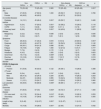
ResultsBaseline characteristicsA total of 93 patients with CKD and 93 age and sex matched patients without CKD were included in the study. Among the CKD group, 38 patients (40.8%) underwent maintenance HD and 55 patients (59.2%) suffered stage 3–5 CKD. A comparison of the demographic characteristics and clinical findings between non-CKD and CKD patients are detailed in Table 1. The median age of entire cohort was 70 years (25–92 years) and 50.5% were male. The age of nondialysis CKD patients were significantly higher than those of HD patients [72 (25–92) years vs 62 (25–88) years, respectively, p=0.001]. Patients with CKD had higher prevalence rate of HT, DM, CHD, and chronic obstructive pulmonary diseases compared to the patients without CKD (p<0.005, respectively). Notably, 64.5% of non-CKD patients with COVID-19 did not have any comorbidity.
Comparison of demographic and clinical characteristics between CKD and non-CKD patients with COVID 19.
| Non-CKD(n=93) | CKD(n=93) | p | Non-dialysis CKD(n=55) | CKD on dialysis(n=38) | p | |
|---|---|---|---|---|---|---|
| Age (years) | 70 (25–92) | 70 (25–92) | 0.983 | 72 (25–92) | 62 (25–88) | 0.001* |
| Sex | ||||||
| Male | 47 (50.5) | 47 (50.5) | 1.000 | 29 (52.7) | 18 (47.4) | 0.766 |
| Female | 46 (49.5) | 46 (49.5) | 26 (47.3) | 20 (52.6) | ||
| Co-morbid diseases | ||||||
| Diabetes mellitus | 14 (15.1) | 45 (48.4) | 0.001* | 29 (52.7) | 16 (42.1) | 0.426 |
| Hypertension | 5 (5.4) | 47 (50.5) | 0.001* | 32 (58.2) | 15 (39.5) | 0.118 |
| COPD | 1 (1.1) | 9 (9.8) | 0.022* | 6 (11.1) | 3 (7.9) | 0.877 |
| Coronary heart disease | 8 (8.6) | 35 (37.6) | 0.001* | 24 (43.6) | 11 (28.9) | 0.223 |
| Malignancy | 2 (2.2) | 4 (4.5) | 0.638 | 2 (3.6) | 2 (5.9) | 0.635 |
| CVO | 3 (3.2) | 6 (6.7) | 0.63 | 5 (9.1) | 1 (2.9) | 0.398 |
| Symptoms | ||||||
| Fever | 28 (30.4) | 31 (33.3) | 0.672 | 8 (14.5) | 23 (60.5) | 0.001* |
| Dyspnea | 40 (43.5) | 44 (47.3) | 0.601 | 30 (54.5) | 14 (36.8) | 0.142 |
| Cough | 36 (39.1) | 39 (41.9) | 0.698 | 22 (40) | 17 (44.7) | 0.809 |
| Fatigue | 18 (19.6) | 17 (20) | 1.000 | 12 (21.8) | 5 (16.7) | 0.777 |
| Myalgia | 7 (7.6) | 2 (2.2) | 0.172 | 1 (1.8) | 1 (2.7) | 1.000 |
| Headache | 4 (4.3) | 4 (4.6) | 1.000 | 2 (3.6) | 2 (6.3) | 0.623 |
| Sore throat | 16 (17.2) | 3 (3.4) | 0.005* | 2 (3.6) | 1 (2.9) | 1.000 |
| Diarrhea | 10 (10.9) | 10 (10.8) | 1.000 | 7 (12.7) | 3 (7.9) | 0.519 |
| Taste/smell disorder | 2 (2.2) | 3 (3.4) | 0.675 | 3 (5.5) | 0 (0) | 0.294 |
| COVID-19 diagnosis | ||||||
| RT-PCR | ||||||
| Positive | 51 (54.8) | 40 (43.5) | 0.122 | 26 (48.1) | 14 (36.8) | 0.388 |
| CT scan | ||||||
| Normal | 5 (5.4) | 4 (4.3) | 0.747 | 2 (3.6) | 2 (5.3) | 1.000 |
| Mild | 38 (41.3) | 32 (34.4) | 0.334 | 22 (40) | 10 (26.3) | 0.253 |
| Moderate | 34 (37) | 34 (36.6) | 0.955 | 19 (34.5) | 15 (39.5) | 0.790 |
| Severe | 11 (12.1) | 23 (24.7) | 0.043* | 13 (23.6) | 10 (26.3) | 0.960 |
| Low saturation (<92%) | 46 (49.5) | 65 (69.9) | 0.005* | 38 (69.1) | 27 (71.1) | 1.000 |
| Outcomes | ||||||
| Severe disease | 47 (50.5) | 67 (72) | 0.003* | 40 (72.7) | 27 (71.1) | 1.000 |
| ICU care need | 16 (17.2) | 32 (34.4) | 0.012* | 22 (40) | 10 (26.3) | 0.253 |
| Secondary infection | 10 (10.8) | 37 (39.8) | 0.001* | 19 (34.5) | 18 (47.4) | 0.305 |
| Length of stay (days) | 9 (2–40) | 12 (2–57) | 0.001* | 12 (2–57) | 11 (2–31) | 0.161 |
| Mortality | 10 (10.8) | 34 (36.6) | 0.001* | 22 (40) | 12 (31.6) | 0.542 |
Data were expressed as median (IQR) for quantitative variables and n (%) for nominal parameters. COPD, chronic obstructive pulmonary disease. CKD, chronic kidney disease. CVO, cerebrovascular disorders. CT, computed tomography. ICU, intensive care unit. RT-PCR, reverse transcription polymerase chain reaction. RRT, renal replacement therapy. SpO2, oxygen saturation.
Dry cough and dyspnea were the most common symptoms at presentation in both groups but non-CKD patients were more likely to present with sore-throat (p=0.005). In subgroup analysis, fever was more frequently detected in patients on maintenance HD compared to CKD patients not on-dialysis (p<0.001). 48.9% of the patients was diagnosed by a positive RT-PCR and the others had symptoms and chest CT findings compatible with COVID 19. There is no significant difference between either CKD patients and non-CKD patients or CKD subgroups in terms of RT-PCR positivity (p=0.122, and p=0.388, respectively). Compared with subjects in non-CKD group, those in CKD group had higher rate of severe chest CT score (p=0.043). However, no difference was observed between CKD subgroups in terms of chest CT severity. CKD patients had higher risk of severe disease, and mortality compared to non-CKD patients (72% vs 50.5%, p=0.003, 36.6% vs 10.8%, p<0.001, respectively). As expected, the rates of ICU admission and the length of hospitalization were higher in CKD group (p=0.012 and p=0.001, respectively). In subgroup analysis, mortality rate was higher in patients with the non-dialysis CKD compared to the patients on HD but these results did not reach statistical significance (40% vs 31.6%, p=0.542) (Table 1).
Laboratory resultsLaboratory findings at admission are summarized in Table 2. We found that the inflammatory status was significantly elevated in CKD patients compared to non-CKD patients. In this regard, significantly higher levels of leucocytes, neutrophils, NLR, SII, CRP, and PCT were observed in patients with CKD (p=0.018, p<0.001, p<0.001, p<0.001, p=0.015 and p<0.001, respectively). On the contrary, lymphocyte counts and LCR were much lower in CKD group (p<0.001 and p=0.001, respectively). As expected, hemoglobin level was lower in patients with CKD compared to non-CKD patients (p<0.001). Notably, serum levels of D-dimer and ferritin which have been associated with increased disease severity and higher mortality in patients with COVID-19, were significantly higher in CKD group than in those without CKD (p<0.001, respectively).
Comparison of hematological and biochemical parameters of the patients.
| NonCKD(n=93) | CKD(n=93) | p | CKD not on dialysis(n=55) | CKD on dialysis(n=38) | p | |
|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.78 (0.45–1.01) | 3.8 (1.3–13.97) | 0.001* | 1.8 (1.3–6.4) | 7.0 (3.1–13.9) | 0.001* |
| Urea | 32 (14–69) | 95 (50–323) | 0.001* | 80 (50–323) | 111 (50–308) | 0.006* |
| LDH (IU/L) | 288 (117–1602) | 300 (170–918) | 0.649 | 304 (170–690) | 296 (178–918) | 0.516 |
| AST (IU/L) | 29 (10–135) | 23 (10–151) | 0.027* | 28 (11–123) | 18 (10–151) | 0.022* |
| ALT (IU/L) | 21 (5–282) | 15 (2–88) | 0.001* | 15 (7–57) | 14.5 (2–88) | 0.186 |
| D-dimer (mg/mL) | 0.31 (0.04–7.56) | 0.59 (0.06–8.51) | 0.001* | 0.55 (0.1–5.9) | 0.62 (0.06–8.51) | 0.938 |
| Fibrinogen (mg/dL | 497 (284–745) | 477 (203–760) | 0.900 | 472 (282–759) | 494 (203–760) | 0.525 |
| CRP (mg/L) | 40.7 (2–6684) | 74 (2.6–275) | 0.015* | 59 (2.6–275) | 82.45 (9–245) | 0.352 |
| PCT (ng/mL) | 0.06 (0.01–8) | 0.49 (0.02–98) | 0.001* | 0.23 (0.02–98) | 1.22 (0.07–20.2) | 0.001* |
| Ferritin (ng/mL) | 150.4 (9.5–2803) | 385 (10–12264) | 0.001* | 216.75 (10–12264) | 708 (11–9635) | 0.001* |
| Hb (g/dL) | 12.6 (7–132) | 10.9 (5.1–14.3) | 0.001* | 11 (5.1–14.3) | 10.8 (5.2–13) | 0.439 |
| WBC (103/mL) | 5.9 (2.5–755) | 7.2 (2.5–17.6) | 0.018 | 8.2 (2.54–17.63) | 6.005 (2.9–17) | 0.035* |
| Neutrophil (103/mL) | 3.5 (1.1–10.9) | 5.26 (1.5–16.3) | 0.001* | 6.1 (1.5–16.3) | 4.68 (1.94–15.3) | 0.187 |
| Lymphocyte (103/mL | 1.2 (0.42–20) | 0.9 (0.1–4.2) | 0.001* | 1.2 (0.11–4.22) | 0.85 (0.2–2.3) | 0.038* |
| Platelets (103/mL) | 185 (96–480) | 183 (52–547) | 0.251 | 192 (91–547) | 158.5 (52–426) | 0.049* |
| NLR | 2.8 (0.15–13.1) | 5.0 (1.0–61.1) | 0.001* | 4.6 (1.0–61.1) | 5.31 (2.44–30.45) | 0.421 |
| PLR | 146.2 (10.9–19680) | 175 (43.3–1309.0) | 0.058 | 160 (43.3–1309.0) | 180.6 (92.4–655) | 0.522 |
| SII | 528.9 (31.8–3762) | 957.8 (143.1–8810.1) | 0.001* | 957.8 (143.158810.1) | 938.8 (228.4–3988.9) | 0.863 |
| LCR | 30.1 (3.0–2000) | 15.2 (0.5–803.3) | 0.001* | 17.9 (0.5–803.3) | 10.9 (1.1–107.7) | 0.097 |
Data were expressed as median (IQR). AST, aspartate aminotransferase. ALT, alanine aminotransferase. PCT, Procalcitonin. CRP, C-reactive protein. Hb, hemoglobin. WBC, leukocyte. CT, computed tomography. LDH, lactate dehydrogenase. LCR, lymphocyte-C-reactive protein ratio. NLR, neutrophils to lymphocytes ratio. PLR, platelet to lymphocyte ratio. SII, systemic immune-inflammation index.
Since the significant differences in levels of the inflammatory markers were observed between non-CKD patients and CKD patients, a subgroup analysis was performed to identify hematological parameters that could differentially affect mortality among patients with CKD. CKD patients died had higher values of NLR (13.13±11.65 vs. 4.68±2.81, p=0.001), PLR (324.51±292.67 vs. 174.23±81.68, p=0.009) and SII (2403.21±1999.46 vs. 831.68±517.02, p=0.001) and lower level of LCR (27.27±50.34 vs 70.78±145.85, p=0.001) compared to those recovered. There was no statistically significant difference in PLR levels between patients with CKD and those without CKD. However, among patients with CKD, significantly higher level of PLR was observed in CKD patients who died, in comparison to CKD patients who recovered (p=0.009) (Table 3). Given that statistically significant difference was found between dead and recovered CKD patients in term of hematological parameters (NLR, PLR, LCR and SII), the optimal cutoff values were identified by ROC analysis (Table 4, Fig. 1). The cut off point was 5.1 (AUC=0.830; p=0.001; CI:0.744–0.916), 1180.5 (AUC=0.811; p=0.001; CI:0.719–0.904), 187.5 (AUC=0.664; p=0.009; CI:0.540–0.787), 9 (AUC=0.712; p=0.001; CI:0.596–0.828) for NLR, SII, PLR, and LCR, respectively. The ROC curve of NLR and SII gave us the best prediction for distinguishing patients with higher risk of death at an earlier stage. Among these parameters, the smallest area belonged to PLR.
Comparison of blood cell count derived inflammation indexes of CKD patients based on mortality.
| Dead (n=59) | Recovered (n=34) | p | |
|---|---|---|---|
| PLR | 160 (43.4–484.2) | 230.4 (67.3–1309.1) | 0.009* |
| 174.23±81.68 | 324.51±292.67 | ||
| NLR | 4 (1.1–14.7) | 10.1 (2.4–61.2) | 0.001* |
| 4.68±2.81 | 13.13±11.65 | ||
| SII | 693.4 (143.1–2332) | 1864.5 (331.8–8810.2) | 0.001** |
| 831.68±517.02 | 2403.21±1999.46 | ||
| LCR | 18.4 (2.4–803.3) | 6.2 (0.5–254) | 0.001* |
| 70.78±145.85 | 27.27±50.34 |
Data were expressed as median (IQR) and mean±SD. LCR, lymphocyte-C-reactive protein ratio. NLR, neutrophils to lymphocytes ratio. PLR, platelet to lymphocyte ratio. SII, systemic immune-inflammation index.
Receiver operating characteristics (ROC) curves and prognostic accuracy of blood cell count-derived inflammation indexes.
| Diagnostic scan | ROC curve | |||||||
|---|---|---|---|---|---|---|---|---|
| Cut off | Spesifisite | Positive predictive value | Negative predictive value | Area | 95% confidence interval | p | ||
| PLR | ≥187.5 | 61.76 | 66.10 | 51.22 | 75.00 | 0.664 | 0.540–0.787 | 0.009* |
| NLR | ≥5.1 | 85.29 | 66.10 | 59.18 | 88.64 | 0.830 | 0.744–0.916 | 0.001* |
| SII | ≥1180.5 | 67.65 | 79.66 | 65.71 | 81.03 | 0.811 | 0.719–0.904 | 0.001* |
| LCR | ≤9 | 58.82 | 83.05 | 66.67 | 77.78 | 0.712 | 0.596–0.828 | 0.001* |
PLR, platelet to lymphocyte ratio. NLR, neutrophils to lymphocytes ratio. SII, systemic immune-inflammation index. LCR, lymphocyte-C-reactive protein ratio.
Receiver Operating Characteristic Curves (ROC) of blood cell count derived inflammation indexes and their respective areas under the curves (AUC) for in hospital mortality. (A) ROC curves of PLR (AUC=0.664) for in hospital mortality. (B) ROC curves of NLR (AUC=0.830). (C) ROC curves of SII (AUC=0.811). (D) ROC curves of LCR (AUC=0.712).
To identify the factors that may affect mortality rate of COVID-19 among CKD patients, we obtained the crude odds ratio (OR) after conducting the logistic regression analysis (Table 5). Step-wise variable selection led to a model with age (>72 years) (1051 [95% CI: 0.998–1.108]; p=0.06), NLR (≥5.1) (3.148 [95% CI: 0.810–12.234]; p=0.098), SII (≥1180.5) (4.419 [95% CI: 1.303–14.992]; p=0.017) and LCR (≤9) (4.984 [95% CI: 1.466–16.945]; p=0.01) as predictors for survival. Conversely, gender, comorbidities (DM, HT, CHD) and PLR did not correlate with the survival outcome. Our study demonstrated that NLR, SII and LCR can be used as a predictor of mortality among CKD patients with COVID 19.
Logistic regression analysis of independent variables associated with in hospital mortality.
| p | ODDS | 95% confidence interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 0.060 | 1.051 | 0.998 | 1.108 |
| Gender | 0.668 | 0.773 | 0.238 | 2.508 |
| HT | 0.263 | 0.487 | 0.138 | 1.716 |
| DM | 0.153 | 2.470 | 0.714 | 8.546 |
| CHD | 0.929 | 0.946 | 0.276 | 3.245 |
| PLR (≥187.5) | 0.657 | 0.722 | 0.171 | 3.048 |
| NLR (≥5.1) | 0.098 | 3.148 | 0.810 | 12.234 |
| SII (≥1180.5) | 0.017* | 4.419 | 1.303 | 14.992 |
| LCR (≤9) | 0.010* | 4.984 | 1.466 | 16.945 |
HT, hypertension. DM, diabetes mellitus. CHD, coronary heart disease. PLR, platelet to lymphocyte ratio. NLR, neutrophils to lymphocytes ratio. SII, systemic immune-inflammation index. LCR, lymphocyte-C-reactive protein ratio. ODDS, odds ratio.
This study is important because, it is the first study to investigate association between SII and disease mortality in CKD patients with SARS-CoV-2 infection. Recently, SII has been shown to be a potential indicator of survival in COVID 19.12 We believe our study may extend the relevance of SII to predicting in-hospital mortality of COVID-19.
Viral nucleic acid test by RT-PCR assay plays a vital role in diagnosis and isolation of individuals with COVID 19. However, lower sensitivity, insufficient stability, and long processing time were detrimental to the control of the pandemic. In the current study, nearly half of the study population were diagnosed based on RT-PCR test. RT-PCR negative patients were only included if the clinical and CT findings were strongly suggestive of COVID 19. Since the previous studies demonstrated that the sensitivity of CT for diagnosis of COVID-19 infection was higher compared with RT-PCR sensitivity,14,15 chest CT was used at the first-line evaluation of the patients with a high clinical probability of COVID-19 pneumonia for rapid diagnosis, isolation and administration of appropriate treatment.
To date, several studies have reported worse clinical outcomes, including more ICU admissions and higher mortality rate among CKD patients with COVID-19.16,17 In agreement with previous reports, the current study showed that mortality was significantly higher in CKD patients than in those without CKD (36.6 vs 10.8%). This may be explained by a pro-inflammatory state with functional defects in the natural and adaptive immunity. Although the highest mortality rate was observed in non-dialysis CKD group compared to HD group, it didn’t reach statistical significance. This difference may be attributed to older age of nondialysis-CKD patients.
Evidence from the global outbreak showed that individuals with older age, male gender and CKD associated morbidities such as HT, DM and CHD are at much greater risk of dying from COVID 19.18–20 On the contrary, the distribution of risk factors for COVID-19 mortality was differed in patients with CKD from the general population. Previous studies have suggested that some commonly reported comorbidities including HT, DM, chronic lung disease and CHD had no influence on mortality among CKD patients with COVID 19.21–23 On the other hand, some studies conducted on hemodialysis patients with COVID 19 reported that only heart failure, CHD and lung disease were risk factors for worse outcome.24 In agreement with COVID-19 database analysis of Turkish Society of Nephrology and European Renal Association,21,22 we found that male sex, HT, CHD and DM do not confer an independent increased risk of mortality. There were conflicting reports whether CKD is a risk factor for death in COVID 19. While initial reports failed to assess the impact of CKD on severity of COVID 19, OpenSAFELY project showed that the top three risk categories for death from COVID 19 were, in order from the highest to the lowest risk, dialysis patients (aHR 3.69), transplant recipients (aHR 3.53) and CKD (aHR 2.52 for patients with eGFR<30mL/min/1.73m2, CKD Grade 4–5).5 This finding emphasize the immunosuppressive nature of the uremic milieu in CKD patients, resulting in increased vulnerability to hyperinflammation and cytokine storm upon SARS-CoV-2 infection, eventually severe disease and death.
Cytokine storm has been linked to severity in COVID 19. A rapid and coordinated innate immune response is the first line of defense mechanism against viral infections. However, when the immune response is dysregulated, it leads to excessive systemic inflammation, and even cause death.25 Previous researches on non-CKD patients with COVID-19 proposed several biomarkers for severe disease, including lymphopenia and increased levels of CRP, LDH, PCT and cytokines (IL-6, IL-10 and tumor necrosis factor), emphasizing the role of the immuno-inflammatory responses in the pathogenesis and progression of COVID-19.26,27 Similarly, in our cohort, higher leukocyte and neutrophil count as well as lower lymphocyte count were observed in CKD patients who died. Blood cell count-derived inflammation indexes, including NLR and PLR have been reported to be a more sensitive biomarker of inflammation than the individual levels of blood cell line.28 Up to now, the potentials of blood cell count-derived inflammation indexes as a predictor of mortality in CKD patients with COVID 19 have been assessed in a few reports. Davila-Collado et all have analyzed the impact of NLR, monocyte to lymphocyte ratio (MLR), and PLR on 37 CKD patients with SARS-CoV-2 infection and noticed that only an elevation in MLR was consistently correlated with increased mortality among patients with CKD.29 In a report of 10 maintenance HD patients, NLR and LCR were associated with the severe form and mortality of COVID 19.30 Another study conducting on 62 HD patients showed that higher NLR was associated with the most severe form of COVID-19.31 In line with the literature, mortality was associated with a lower LCR but higher NLR in our study group. It was also remarkable that SII was significantly higher in CKD cases developing mortality. To the best of our knowledge, to date, no study has been carried out to evaluate the feasibility of SII to assess COVID-19 disease mortality in CKD patients.
As a new systemic inflammation indicator, SII, based on lymphocyte, neutrophile, and platelet counts has been reported as prognostic factor in COVID 19.12 Utility of SII to identify COVID 19 patients at higher risk of death is given by the differential roles that lymphocyte, neutrophil and platelet playing during immune response. Lymphocytes are known to be responsible for eliminating virus infected cells.32 Although neutrophils are the most important cellular defense against infections, it is not clear whether neutrophils play a role in anti-viral defense in viral pneumonia.33 However, neutrophil recruitment into the lungs has been observed only in pneumonia patients with ARDS, support that neutrophils play a role defending the airway epithelium in the presence of severe SARS-CoV-2 virus infection.33 Platelets contribute to hemostasis and also participate in the inflammation and host defense. Decreased platelet production and increased consumption due to diffuse alveolar damage are thought to cause thrombocytopenia in COVID-19 patients.34,35 In consideration of these factors, SII might be better able to reflect the balance of host inflammatory and immune status in COVID 19. The current study revealed that the discriminative performance of SII and NLR were the highest among the hematological indexes evaluated, in predicting disease mortality. There seems to be some evidence to indicate that SII was not inferior to NLR which is widely used to predict the severity of COVID-19 disease.36 Our results are in line with recently published studies identifying the value of SII to predict the risk of in-hospital mortality of COVID-19 patients and confirms the reliability of SII as a powerful predictor of survival.37,38 As SII is based on the results of complete blood count analysis, it is inexpensive, more simple, easily applicable and more suitable for widespread use. Quantification of SII at admission would guide the physician for early identification and timely management of the patients with worse survival.
In addition, we found that the AUC was significant with the PLR and LCR. Qu R. et al. reported that the PLR may predict mortality in COVID-19 patients.39 Although PLR, which increase thrombosis development and responsible for the cytokine and chemokine cascade, was significantly higher in CKD patients developing mortality, it didn’t predict disease mortality.40 It can be explained by the relatively low number of the patients. As such, Fois et al. observed higher value of PLR in deceased patients with COVID 19. However, after adjusting for confounders, they found a borderline significance between worse survival and PLR (p=0.058).37 Since the SARS-CoV-2 viral load has been highly correlated with lymphocyte count and CRP value, LCR can help to predict disease severity.41 In the current study LCR showed a reasonable ability to predict disease mortality.
There were several limitations to our study that warrant consideration. First, it was a retrospective, single-center study of CKD patients with COVID 19 admitted to the hospital. Large- scale multicenter prospective studies should be performed to support our findings. Second, patients without a positive RT-PCR were also included in the study but all RT-PCR-negative patients had clinical features and chest CT findings strongly suggestive of COVID-19.
ConclusionsWe report for the first time that SII is able to distinguish COVID-19 infected CKD patients of worse survival and it is as powerful as NLR in this regard. Since SII can be easily quantified from blood sample data, it can provide significant benefits for early identification and timely management of CKD patients with worse survival.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare that they have no conflict of interest.
We would like to thank Emire Bor, EMPIAR Statistics, for performing statistical analysis of the study and to María Teresa Ferrero Gil for her valuable help in translating the article into Spanish.