Autosomal recessive polycystic kidney disease (ARPKD) is a rare hereditary disease with an estimated incidence of 1:10,000 to 1:40,000.1,2 It is due to a mutation in the PKHD1 gene that codes for a protein called fibrocystin or polyductin which is responsible for differentiating between kidney and bile duct tubules.1 It is characterised by kidney cysts with progressive kidney function deterioration and biliary dysgenesis that causes congenital liver fibrosis.2,3 Subtypes can be established according to the age of presentation and severity of the disease: prenatal, neonatal, childhood and young adult.3 The most severe cases with nephromegaly and Potter's syndrome are described in the first year of life,4 while those detected in adolescence are less symptomatic and kidney function deteriorates later. Nevertheless, there are few published articles describing patients that were diagnosed as adults.
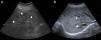
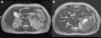
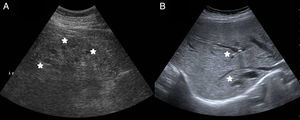
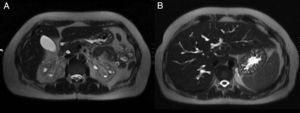
A 39-year-old woman with no relevant medical history was seen in the emergency room for cramping in her legs during the last 6 months, but that had been more severe during last week. It was associated with asthenia, hyporexia, and 20kg of weight loss during the last year. She denied taking NSAIDs or other drugs. No fever, photosensitivity, skin lesions, arthralgia, or other symptoms of systemic disease. Physical examination was normal, except for a blood pressure of 93/61mmHg. The blood tests showed creatinine 5.53mg/dl, urea 386mg/dl, sodium 127mEq/l, potassium 5.3mEq/l, magnesium 1.2mg/dl, calcium 6mg/dl, PTH 672pg/ml, haemoglobin 8.4g/dl, pH7.28, bicarbonate 13.6mmol/l, pCO2 29mmol/l and anion GAP 19. Urinalysis: proteinuria 310mg/day, natriuria 186mEq/day, calciuria 86mg/day, magnesuria 48mg/day, with no glycosuria, haematuria, or leukocyturia. Anaemia and smear tests were normal. Immunological and serum test were normal. The TSH, cortisol, ACTH, and ADH hormones were normal, but renin and aldosterone were elevated. Abdominal ultrasound: kidneys decreased in size with poor corticomedullary differentiation, cortex thinning, and predominant bilateral simple medullary cysts under 1cm, with no lithiasis or hydronephrosis (Fig. 1). Cystic dilations of the intrahepatic bile ducts were observed as an incidental finding on the ultrasound and confirmed with magnetic resonance cholangiopancreatography, which are indicative of Caroli's disease (Fig. 2).
After intravenous magnesium, calcium, potassium, and bicarbonate treatment, the cramps resolved. She was prescribed salt supplements to normalise her blood pressure. At discharge: creatinine 4.5mg/dl, sodium 133mEq/l, potassium 3.7mEq/l, calcium 7.1mg/dl, magnesium 2.2mg/dl, pH7.34 and bicarbonate 24mmol/l. Oral salt supplements were considered necessary. Family screening of her parents, siblings, and children, with kidney ultrasound and blood tests was normal. There was no genetic relationship between her parents. The confirmatory diagnosis was obtained using genetic testing that detected the mutation in the PKDH1 gene; furthermore, the presence of associated tubulopathy was ruled out. After four months, the patient began peritoneal dialysis.
ARPKD is a paediatric disease with kidney and liver symptoms, with few cases published of adult-onset. The differential diagnosis was first made as autosomal dominant polycystic kidney disease (ADPKD) that, although rarely, can sometimes be associated with Caroli's disease.5 It was discarded because her kidneys were not increased in size and the kidney cysts were sub-centimetre in size. Other hereditary cystic nephropathies were also excluded: cystic medullary kidney disease, nephronophthisis, disease caused by a mutation in the HNF1b gene, tuberous sclerosis, Von Hippel-Lindau disease, as well as acquired cystic kidney disease: simple cysts, acquired cystic disease, or Cacchi-Ricci disease.
In our case, the tubular changes, hypomagnesaemia, natriuresis, and polyuria, with cramping symptoms were the guiding symptoms. Although associated electrolyte changes have been described,6–8 this is the first case of ARPKD in the literature that started with symptomatic hypomagnesaemia. Moreover, other concomitant tubulopathies, such as Gitelman syndrome that could explain the findings of hyperreninism and hyperaldosteronism secondary to hypomagnesaemia, were ruled out by the genetic testing. In our patient, these could be due to the increased urinary excretion of sodium, water, and magnesium due to disfunctioning tubular cells.
There is a progressive deterioration of kidney function and more than half of the patients need renal replacement therapy before the age of 20 years.1,9 Hypertension is a factor of poor prognosis, it starts during the first months of life and improves with age.2,7 The patient did not present with a history of hypertension and during admission she had low blood pressure, probably due to her salt-wasting nephropathy, which could have favoured the benign progression of this case.
In conclusion, ARPKD is a disease of the childhood, but it should not forgotten that it can start in adults with more moderate clinical manifestations. This disease should be suspected in adults with end-stage chronic kidney disease, radiological findings that are compatible with the diagnosis (millimetre medullary kidney cysts, dilation of the intrahepatic bile ducts), and associated symptomatic electrolyte disorder.
Please cite this article as: Martínez V, Trasancos C, Ramos F, Alcázar C, Cabezuelo JB, García M. Poliquistosis renal autosómica recesiva diagnosticada en mujer de 39 años con fallo renal y calambres. Nefrologia. 2016;36:318–320.