This study's objective is to evaluate the correlation relationship between Podocalyxin (PCX), an urinary marker of podocytes, urinary albumin-creatinine ratio (ACR) and the predictive value of PCX in the routine screen of early diabetic kidney disease (DKD) among older people. We also aimed to explore its prediction value despite of other metabolic factor and how PCX alters in the predictive power for early stage of diabetic nephropathy. In retrospective, 320 cases of older patients diagnosed with type 2 diabetes mellitus who met both inclusion and exclusion criteria were collected and divided with levels of urinary albumin, that is, normal albuminuria group, microalbuminuria group and healthy group. The correlation coefficient between PCX and ACR, and the odds ratio of PCX were gauged in the study. Area under the receiver operating characteristic (ROC) curve was also calculated. There were 188 patients in the normal group with urine ACR<30mg/g, and 132 patients in the microproteinuria group with urine ACR 30–300mg/g. 132 cases of DKD diagnosed with ACR, among them, 104 cases of DKD were predicted by PCX. The percentage correction value was 78.8%. The following parameters such as gender, age, course of disease, glycated hemoglobin, triglyceride, total cholesterol, BMI, blood pressure, uric acid, and eGFR were used as variables for adjustment to establish the prediction model of urine PCX and ACR. Multiple logistic regression test was carried out to evaluate against the predictive ability of the model. The area under the ROC curve corresponding to the regression model after adjustment is 0.952. Although factors such as the course of disease, HbA1C, UA, and eGFR could influence on the predictive ability of PCX, PCX still has a good ability to predict early DKD in older patients. Therefore, it could be used as a diagnostic indicator for early-stage DKD in older patients.
El objetivo de este estudio es evaluar la relación de correlación entre la podocalyxina (PCX), un marcador urinario de podocitos, el cociente albúmina-creatinina urinaria (ACR) y el valor predictivo de PCX en el cribado rutinario de la enfermedad renal diabética temprana (ERC) en personas mayores.. También nos propusimos explorar su valor de predicción a pesar de otros factores metabólicos y cómo la PCX altera el poder predictivo de la nefropatía diabética en la etapa temprana. En retrospectiva, se recogieron 320 casos de pacientes mayores diagnosticados con diabetes mellitus tipo 2 que cumplían con los criterios de inclusión y exclusión y se dividieron con los niveles de albúmina urinaria, es decir, grupo de albuminuria normal, grupo de microalbuminuria y grupo sano. El coeficiente de correlación entre PCX y ACR, y la razón de posibilidades de PCX se midió en el estudio. También se calculó el área bajo la curva de característica operativa del receptor (ROC). Hubo 188 pacientes en el grupo normal con ACR en orina <30 mg /gy 132 pacientes en el grupo de microproteinuria con ACR en orina 30-300 mg /g. 132 casos de DKD diagnosticados con ACR, entre ellos 104 casos de DKD fueron predichos por PCX. El valor de corrección porcentual fue del 78,8%. Los siguientes parámetros como sexo, edad, curso de la enfermedad, hemoglobina glucosilada, triglicéridos, colesterol total, IMC, presión arterial, ácido úrico y TFGe se utilizaron como variables de ajuste para establecer el modelo de predicción de PCX y ACR en orina. Se realizó una prueba de regresión logística múltiple para evaluar la capacidad predictiva del modelo. El área bajo la curva ROC correspondiente al modelo de regresión después del ajuste es 0,952. Aunque factores como el curso de la enfermedad, HbA1C, UA y eGFR podrían influir en la capacidad predictiva de PCX, PCX todavía tiene una buena capacidad para predecir la DKD temprana en pacientes mayores. Por lo tanto, podría usarse como un indicador de diagnóstico para la ERD en estadio temprano en pacientes mayores.
Non-communicable diseases (mainly including diabetes mellitus, hypertension, tumors, cardiovascular and cerebrovascular diseases) are the main causes of death. 63% of global casualties each year are caused by non-communicable diseases, and 80% of mortality in China each year are owing to noncommunicable diseases.1 The 21st century is witnessing a rapid growth in the aging of China's population. Among them, more than 20% of the older people are diabetic patients, and more than 45% of the older people are in the state of prediabetes. DKD refers to kidney disease caused by diabetes. In China, the incidence of DKD in diabetic patients accounts for about 20–40%. DKD has also become the main cause of end-stage renal disease.2,3
Confirmation of the diagnosis in time and intervention in its early stages can ameliorate the occurrence and progression of DKD, further reduce the risk of cardiovascular disease, and reduce mortalities caused by from noncommunicable diseases. The incidence of diabetic kidney disease diagnosed according to the presence of microalbuminuria (mAlb) or proteinuria is about 5–20% in T1DM patients and about 25–35% in T2DM. Extensive studies demonstrate that proteinuria, especially microalbuminuria, lacks sensitivity and specificity in the diagnosis of DKD, and the value of urinary protein is greatly limited in the prognosis of renal function. Studies have shown that 10% of diabetic patients with early progressive renal failure have normal urine albumin,4 and the level of urine mAlb can return to normal without treatment and intervention.4,5 Current research has confirmed that podocytes play a key role in the early stage of DKD, and the detection of urinary markers excreted by podocyte has received increasing research interest in the diagnosis of DKD.
Podocalyxin (PCX) is a transmembrane protein which is located to the apical wall of glomerular podocytes.6 The function of PCX is to maintain the shape and slit diaphragm of podocyte.7 Hara et al. reported that PCX shed from injured podocytes into urine, as small vesicles that appear on the tip of microvilli in podocytes.8 The levels of urinary PCX (u-PCX) increased significantly in patients with diabetic nephropathy. In a previous study, the level of u-PCX was high in diabetic nephropathy, but there were only 9 cases of diabetic nephropathy, and no further analysis were performed for the significance of u-PCX as a diagnostic marker of DN to differentiate from other glomerular kidney diseases.9
According to a study published in the New England Journal of Medicine in 2014, it reported that the incidence of complications such as acute myocardial infarction, stroke, amputation, and hyperglycemia in adult diabetic patients showed a downward trend. However, the incidence of End-stage renal disease (ESRD) has not decreased significantly, suggesting that DKD is still a major disease that seriously threatens human health in the current time, thus requires our special attention.10 Therefore, it is important to identify DKD in its early stages as prompt treatment can reduce the medical and economic burden of this disease, especially in older population, because older patients take stakes of more cardiovascular and cerebrovascular risks, so that early detection and identification is especially important.11 This article aims to explore the correlation between urine PCX and ACR by analyzing the routine screening parameters of the older people in local community, and to provide a novel diagnostic indicator target for the early stage of DKD.
Materials and methodsPatientsPatients who met the inclusion and exclusion criteria after routine physical examinations were collected from January 2017 to November 2019 in the residents of six community in Suzhou New District. At the same time, 30 healthy patients were selected and enrolled in the group as normal control. Inclusion criteria: (1) meet the criteria of diagnosis and classification of type 2 diabetes mellitus proposed by the World Health Organization in 1999, according to the 2016 expert consensus on clinical diagnosis of diabetic kidney disease in Chinese adults12; (2) No history of diabetic kidney disease (urine ACR<30mg/g); (3) Early diabetic kidney disease (urine ACR 30–300mg/g); (4) age≥60 years.
Exclusion criteria: (1) Patients with severe organ dysfunction such as heart, liver, kidney, and others. The criteria for evaluating organ dysfunction was put as following: cardiac function grade III or higher NYHA, chronic kidney disease stage 3 or higher (according to the criteria by Kidney Diseases Improving Global Outcomes, KDIGO), alanine aminotransferase ALT above the upper limit of normal 2.5 times; (2) comorbidities such as hypertension; (3) type 1 diabetes, special types of diabetes and gestational diabetes; (4) acute complications such as ketoacidosis, hypertonic coma, infection and other states of stress. The patients were divided into group with normal ACR levels according to ACR (Urinary ACR<30mg/g), trace proteinuria group (urine ACR 30–300mg/g), and a control group of 30 normal healthy people. This study was approved by the ethics committee of the People's Hospital of Suzhou New District. All Participants received clear instructions from the investigators about the study and signed the written form of informed consent.
Data collectionThe clinical data included patient gender, age, course of diabetes, height, weight, and body mass index (BMI=weight/height 2); the estimated glomerular filtration rate (eGFR) was calculated based the CKD-EPI formula. All the patients started fasting at 20:00 on the previous day of blood sample collection. The venous blood was collected in the next morning and daytime during the fasting state, and the supernatant of blood sample was centrifuged and resuspended in a common procoagulant test tube. At the same time, 40mL of morning urine was obtained, divided into two separate glasses, and one glass was used for urine detection including routine urine test combined with ACR, Another collection of urine sample was centrifuged and the supernatant was collected and resuspended in another cup, then stored at −80°C. The automatic biochemical immunoanalyzer (abort cl16200, Roche, Germany) in the laboratory of our hospital was used for the test of blood glucose, glycated hemoglobin, cholesterol, triglycerides, uric acid, creatinine, albumin, urine albumin, urine creatinine. The instruments of automatic urine analysis were utilized to detect urine routine. The expression level of urine PCX was measured with a commercial enzyme-linked immunosorbent assay kits (MyBioSource, Inc. San Diego, CA, USA), and the experiments were performed according to the protocol provided by the manufacturer. In order to eliminate the effects of confounding factors such as of urine concentration or dilution on the results, all measurements from the urine were presented after the results were adjusted as the ratio of measured values to Ucr.
Model establishment and verificationSpearman correlation analysis was used to evaluate the correlation strength between PCX and ACR; combined with clinical parameters age, gender, disease course, the metabolic factors (BMI, FPG, HbAlC, TG, TC, UA, BMI) that may affect PCX. These data were included in the adjusted variables. Taken together, these data were utilized to establish multiple factor regression model to analyze the correlation strength of PCX to ACR after adjustment. The area under the receiver operating characteristic (ROC) curve was calculated for the estimation of the discriminative degree of the prediction model. The criteria of ACR≥30mg/g was used as the threshold to calculate the Youden's index of the ROC curve. The higher the index, the greater the sensitivity and specificity of the diagnosis; Hosmer–Lemeshow (HL) chi-square test was used to assess the calibration degree of the prediction model. P>0.05 is considered as of no statistically significance.
Statistical analysisSPSS 21.0 software was used for statistical analysis. All data were tested against normality distribution. The measurement data was expressed as x¯±s. Comparison between groups was performed using t test. Measured data of non-normal distribution was expressed as median (P25, P75). The comparison between two groups used the Kruskal–Wallis test for non-parametric data, the comparison between multiple groups of means uses the one-way ANOVA test, and the correlation test between the two groups of non-normally distributed data uses Spearman correlation analysis. Sensitivity and specificity were analyzed using receiver operating characteristic (ROC) curves. The difference was statistically significant if P value is less than 0.05.
ResultsPatient indicatorsA total of 320 eligible patients were enrolled in our study during the period, including 156 males and 164 females. There were 188 patients in the normal group with urine ACR<30mg/g, and 132 patients in the microproteinuria group with urine ACR 30–300mg/g. The clinical information of the two groups are shown in Table 1.
Clinical and laboratory data of the studied group.
| Clinical and laboratory characteristics | Healthy controls (n=30) | Normoalbuminuria (n=188) | Microalbuminuria (n=132) | F/χ2 | P value |
|---|---|---|---|---|---|
| Gender | – | – | – | 0.178 | 1.026 |
| Male, n (%) | 14 (46.67) | 93 (49.47) | 63 (47.73) | – | – |
| Female, n (%) | 16 (53.33) | 95 (50.53) | 69 (52.27) | – | – |
| Age (years) | 67.03±1.67 | 68.86±1.56b | 74.08±2.685a,b | 299.258 | 0.000 |
| DM duration (years) | – | 9 (7.25,13) | 13 (12, 14) | 74.251 | 0.000 |
| BMI (kg/m2) | 21.89±1.57 | 21.88±1.26 | 21.77±1.25 | 0.332 | 0.718 |
| Systolic blood pressure (mmHg) | 125.03±12.36 | 129.34±11.74 | 130.69±13.15 | 0.856 | 0.153 |
| Diastolic blood pressure (mmHg) | 75.32±5.08 | 78.68±7.47 | 82.59±8.57 | 0.965 | 0.121 |
| FBG (mmol/L) | 5.64±0.59 | 5.90±1.02 | 6.00±1.01 | 1.702 | 0.184 |
| ALB (mmol/L) | 39.91±2.68 | 40.77±3.30 | 40.53±2.22 | 1.227 | 0.294 |
| eGFR (mL/min/m2) | 93.32±1.64 | 86.23±6.72b | 82.37±5.72a,b | 43.583 | 0.000 |
| TC (mmol/L) | 5.35±0.23 | 5.24±0.33 | 5.26±0.32 | 1.380 | 0.253 |
| TG (mmol/L) | 1.34±0.16 | 1.38±0.18 | 1.36±0.18 | 0.979 | 0.377 |
| HbA1c (%) | 5.87±1.01 | 6.73±0.63b | 6.90±0.58a,b | 29.764 | 0.000 |
| UA (μmol/L) | 394.03±74.03 | 386.6±74.89 | 413.48±64.47a | 5.585 | 0.004 |
| PCX/Ucr (μg/g) | 0.59 (0.51, 1.56) | 1.59 (0.96, 2.36)b | 8.05 (3.71, 15.21)a,b | 227.644 | 0.000 |
| ACR (mg/g) | 4.57 (3.48, 5.33) | 14.33 (9.70, 20.80)b | 96.95 (80.30, 113.23)a,b | 275.987 | 0.000 |
BMI, body mass index; FBG, fasting blood glucose; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; TC, total cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; PCX, podocalyxin; UA, uric acid; ACR, Albumin Creatinine Ratio; Ucr, urinary creatinine.
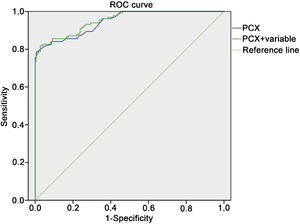
According to the results of Spearman correlation analysis, the data suggested that the correlation coefficient between PCX and ACR is 0.852. Based on the analysis of ROC curve, the area under the ROC curve of PCX is 0.946, cutoff is 3.09. Finally, the sensitivity and specificity are 0.84, and 0.91, respectively (Fig. 1). 132 cases of DKD diagnosed with ACR, among them, 104 cases of DKD were predicted by PCX. The percentage correction value was 78.8% (data not shown).
Receiving Operator Characteristics (ROC) curve for the diagnosis of early-stage diabetic kidney disease using urinary PCX. The abscissa represented specificity, the ordinate represented sensitivity, and the area under the curve represented the diagnostic value of urinary PCX in the early stage diabetic kidney disease.
A univariate binomial logistic regression model was established in which the PCX is set as independent variable and the presence of microlbuminuria or proteinuria as the dependent variable, and the value of odds ratio (OR) ACR was 6.092. The parameter Eger is also enrolled into the analysis of multiple factor logistic regression model. After adjustment, the value of OR for ACR was 5.742 (Table 2). The results demonstrated that the correlation coefficient between PCX and ACR was underestimated when there were metabolic factors, and the correlation between the two variables was more obvious after eliminating the effects of metabolic factors.
Logistic regression analysis of PCX and ACR before and after adjusting variables.
| Variable adjustment time | OR value | Regression coefficients | Wald | P value | 95% CI |
|---|---|---|---|---|---|
| Before adjustment | 6.092 | 0.306 | 34.773 | <0.01 | 3.341–11.106 |
| After adjustment | 5.742 | 0.314 | 0.580 | <0.01 | 3.101–10.630 |
PCX, podocalyxin ACR, albumine to creatinine ratio.
The H-L chi-square test and the area under the ROC curve were used to test the degree of calibration and discrimination in the regression model before and after adjustment (Table 3). The value of chi-square test in the regression model before adjustment was 16.985, P<0.01, and the area under the ROC curve was 0.946; the value of chi-square for the adjusted regression model test value was 5.952, P<0.01, and the area under the ROC curve was 0.952 (Fig. 1). Taken together, the results illustrated that the degree of calibration and discrimination of the adjusted prediction model is better than the unadjusted regression model.
Analysis of Hosmer–Lemeshow chi-square test for PCX and ACR regression models.
| Variable adjustment time | H-L test chi-square value | P value | Cox & Snell R2 | Nagelkerke R2 | AUC | 95% CI |
|---|---|---|---|---|---|---|
| Before adjustment | 16.985 | 0.000 | 0.573 | 0.781 | 0.946 | 0.924–0.969 |
| After adjustment | 5.952 | 0.000 | 0.580 | 0.790 | 0.952 | 0.931–0.973 |
PCX, podocalyxin ACR, albumine to creatinine ratio.
The aging population has become a prominent problem in the current world. Rapid growth of senior people and its notable accompanying demand for medical care and nursing facilities has posted severe challenges to the families and whole society. According to relevant data, the life expectancy of human settlements reached 76.34 years in China in 2015.12 Aging population and together with the shifts in life styles has aroused emerging numbers of patients with chronic diseases, which has become a major problem of public health that threatens the health of older people. The predicted number of older populations will continue its drastically growth in the next few decades. If the incidence of chronic diseases could not be contained, it would lead to a serious decline in the life quality and rapid rise of morbidity and mortality. The total number of people with diabetes worldwide was estimated to be 451 million in 2017, and it is expected to rise to about 693 million by 2045.13 In China, only 1.1% of patients with chronic kidney disease (CKD) caused by diabetes have been hospitalized, and moreover, CKD by diabetes has become the main cause of ESRD.3 Robles et al. found that the presence of urine microalbumin in approximately 20% of the older people is associated with hypertension.14 The early treatment and intervention could positively reverse the outcome of patients with DKD, and if the treatment is not in time, DKD itself will eventually progress to ESRD, which requires long term Renal Substitutive Therapy and even leading to death. Most older patients with type 2 diabetes often have other complications, especially cardiovascular disease. Many older DKD patients have died of cardiovascular disease before ESRD. Therefore, in the treatment of older DKD patients, control of blood glucose, blood lipids, blood pressure and other risk factors should be considered, in addition to their impact on the life quality. However, presence of urinary albumin is sometimes delayed in DKD. Studies have revealed that the prevalence of diabetic kidney disease diagnosed based on microalbuminuria or proteinuria is about 5–20% in T1DM patients and about 25–35% in T2DM. Renal biopsy is considered only if clinical manifestations are not fully established but with suspicion of diabetic nephropathy. Accumulating studies elucidated that proteinuria, especially microalbuminuria, lacks sensitivity and specificity in the diagnosis of DKD, and the predictive value of urinary protein is greatly confined in the prognosis of renal function. A study, conducted by Dr. Klessens et al., is aimed to investigate the prevalence of diabetic nephropathy in diabetic patients using autopsy results. The results suggest that the prevalence of diabetic nephropathy confirmed by pathological findings is higher than previously expected, indicating the pathological changes of diabetic nephropathy may have existed long before the presence of clinical symptoms.15 Therefore, it is not enough to prevent and treat diabetic nephropathy with the presence of proteinuria as the starting point. Therefore, it is urgent to seek for new targets and new methods for the prevention and treatment of DKD.
During the occurrence and development of diabetic nephropathy, podocytes were confirmed to have suffered functional and structural damage. In patients with diabetic nephropathy, the density and absolute number of glomerular podocytes decreased significantly. When podocytes are damaged, there will be obvious morphological changes. In severe cases, the podocytes will even fall off the basement membrane and be discharged out of the kidney with urine. Podocyte damage is the crucial factor of glomerular disease. The decrease or loss of the podocytes is an important indicator for assessing the degree of the glomerular damage and progression of kidney sclerosis. Detecting the urinary podocytes and its related proteins is a way to understand the severity of the glomerular disease.16 Recent studies have demonstrated that podocytes detachment existed in diabetes patients with normoalbuminuric by analysis on the kidney biopsy samples via electron microscopic morphometric techniques based on kidney biopsy. These findings suggest that detached podocytes and their fragments (marked Podocalyxin) might appear in urine of diabetes patients with normal levels of urinary albumin, and podocalyxin-positive element (PCX+EL) might be a possibly promising marker in the early stage of nephropathy, but its role has remained in veil.17,18 PCX, a negatively charged sialo glycoprotein, expressed in the membrane of podocytes, plays an essential role in maintaining the function of glomerular podocytes.19 Urinary PCX levels were found to be associated with levels of UACR and also increased in diabetic patients with normal levels of urinary albumin,20 which is in consistence with our findings. While PCX may be an excellent marker for indicating podocyte injury. Our study found that there is a good correlation between UPCX/cr and ACR, with a correlation coefficient of 0.852. The area under the ROC curve of UPCX/cr for early diabetic kidney disease is 0.946. In group with microalbuminuria, UPCX/cr was significantly higher than that in the normal group as control. Nevertheless, in the non proteinuric group, the UPCX/cr was still higher than in the healthy control group, suggesting that UPCX/cr may be an earlier indicator than ACR in the surveillance of DKD. Hara et al. used immunofluorescence to detect the expression of PCX in kidney tissue and urine, and found that the increased level of urinary PCX in originated from the apical membrane region of podocytes, rather than shedding fragments from podocytes, indicating that PCX in urine was an earlier indicator than ACR.9 Due to the natural change of kidney structure, accompanied with the functional decline, and reduced ability of damage repair, the incidence of CKD is as high a 24.2–38%.21,22 Chronic inflammatory status in the microenvironment, cardiovascular diseases, hypertension and other metabolic abnormalities are also contributing factors to the ever-increasing incidence of CKD. Our study may shed some light on the gloomy status for the prediction and prognosis of chronic kidney diseases.
In summary, our study demonstrated that the expression level of PCX in the urine could be used as biomarker for the early damage of podocytes in DKD patients. UPCX/cr might become a more sensitive indicator of the glomerular disease damage as compared to proteinuria. As a potential noninvasive test with a good diagnostic value for DKD, this could potentially compensate for the disadvantages of renal biopsy for aging population.
Conflicts of interestsAll the authors declare of no conflicts of interests.
This study was supported by research grants from Suzhou New District Medical and Health Science and Technology Program Foundation (2018Q005),People's Hospital of SND Science Innovation Fund Project (SGY2019B03), Suzhou New District Medical and Health Science and Technology Program Foundation (2016Z008).