Acute kidney injury (AKI) is a frequent complication of hematopoietic stem cell transplantation (HSCT) and appears to be linked to increased morbidity and mortality. The aim of this study was to evaluate the incidence, etiology, predictors and survival impact of early AKI in the post-allogeneic HSCT setting.
Patients and methodsWe performed a retrospective single center study that included 155 allogeneic transplant procedures from June 2017 through September 2019.
ResultsAKI was observed in 50 patients (32%). In multivariate analysis, age (OR 31.55, 95% CI [3.42; 290.80], p=0.002), evidence of disease at the time of transplant (OR 2.54, 95% CI [1.12; 5.75], p=0.025), cytomegalovirus reactivation (OR 5.77, 95% CI [2.43; 13.72], p<0.001) and hospital stay >35 days (OR 2.66, 95% CI [1.08; 6.52], p=0.033) were independent predictors for AKI. Increasing age (HR 1.02, 95% CI [1.00; 1.04], p=0.029), increasing length of hospital stay (HR 1.02, 95% CI [1.01; 1.03], p=0.002), matched unrelated reduced intensity conditioning HSCT (HR 1.91, 95% CI [1.10; 3.33], p=0.022), occurrence of grade III/IV acute graft-versus-host disease (HR 2.41, 95% CI [1.15; 5.03], p=0.019) and need for mechanical ventilation (HR 3.49, 95% CI [1.54; 7.92], p=0.003) predicted an inferior survival in multivariate analysis. Early AKI from any etiology was not related to worse survival.
ConclusionPatients submitted to HSCT are at an increased risk for AKI, which etiology is often multifactorial. Due to AKI incidence, specialized nephrologist consultation as part of the multidisciplinary team might be of benefit.
La lesión renal aguda (LRA) es una complicación frecuente del trasplante de células madre hematopoyéticas (TCMH) y parece estar asociado a un incremento en la morbilidad y la mortalidad. El objetivo de este estudio fue evaluar la incidencia, la etiología, los factores predictivos y el impacto en la supervivencia de la LRA temprana en el contexto posterior al TCMH alogénico.
Pacientes y métodosSe realizó un estudio retrospectivo en un único centro que incluyó 155 procedimientos de trasplante alogénico desde junio de 2017 hasta septiembre de 2019.
ResultadosSe observó LRA en 50 pacientes (32%). En el análisis de múltiples variables, la edad (OR 31,55, IC del 95% [3,42; 290,80], p=0,002), la evidencia de enfermedad en el momento del trasplante (OR 2,54, IC del 95% [1,12; 5,75], p=0,025), reactivación de citomegalovirus (OR 5,77, IC del 95% [2,43; 13,72], p<0,001) y estancia hospitalaria>35 días (OR 2,66, IC del 95% [1,08; 6,52], p=0,033) fueron los factores predictivos independientes para LRA. La mayor edad (HR 1,02, IC del 95% [1,00; 1,04], p=0,029), la mayor duración de la estancia hospitalaria (HR 1,02, IC del 95% [1,01; 1,03], p=0,002), TCMH con acondicionamiento de intensidad reducida no relacionados emparejados (HR 1,91, IC del 95% [1,10; 3,33], p=0,022), aparición de enfermedad injerto contra huésped aguda de grado iii/iv (HR 2,41, IC del 95% [1,15; 5,03], p=0,019) y necesidad de ventilación mecánica (HR 3,49, IC del 95% [1,54; 7,92], p=0,003) predijeron una supervivencia inferior en el análisis de múltiples variables. La LRA temprana de cualquier etiología no se asoció con una peor supervivencia.
ConclusiónLos pacientes sometidos a TCMH presentan un mayor riesgo de LRA, cuya etiología es con frecuencia multifactorial. Debido a la incidencia de LRA, la consulta a un nefrólogo especializado como parte del equipo multidisciplinario podría ser beneficiosa.
Hematopoietic stem cell transplantation (HSCT) is increasingly used to treat several malignant and non-malignant hematologic diseases and some solid neoplasms. Acute leukemia represents more than 50% of allogeneic transplant indications, while lymphoproliferative disorders and multiple myeloma are the most frequent for autologous transplants.1–3
The allogeneic HSCT procedure is complex and the selection of conditioning regimen, either myeloablative (MAC) or of reduced intensity (RIC), type of donor, and stem cell source all influence the expected outcome.1,4
Both acute kidney injury (AKI) and chronic kidney disease (CKD) are described as frequent complications of HSCT and they appear to be linked to increased morbidity and mortality.5,6
The incidence of AKI depends on the type of HSCT, conditioning regimen and the definition used to classify AKI, and usually occurs within the first 100 days after transplantation. While AKI incidence ranges from 10% to 20% in autologous HSCT, it can vary from 50% in RIC to 75% in MAC allogeneic transplants. Timing and severity of AKI appear to correlate with mortality, and the need for dialysis results in a bad outcome (mortality rate of 80–100%).7–10 Therefore, efforts have been made to identify, prevent and treat AKI early.
The etiology of AKI after allogeneic HSCT can be divided into three categories for better understanding: pre-renal, renal, and post-renal (obstructive). Pre-renal causes include mainly dehydration due to GI losses and mucositis. Renal causes encompass glomerular affections like transplantation-associated thrombotic microangiopathy (TA-TMA); acute tubular necrosis (ischemic and/or toxic) due to sepsis, septic shock, and dimethyl sulfoxide toxicity from cryopreserved stem cells; and acute interstitial nephritis induced by drugs or opportunistic infections like BK virus or adenovirus. Obstructive causes may be intratubular (e.g. acyclovir) or postrenal secondary to retroperitoneal fibrosis, lymphadenopathy, or hemorrhagic cystitis due to BK or adenovirus.10
The aim of this study was to evaluate the incidence, etiology, predictors and survival impact of AKI in the post-allogeneic HSCT setting.
Material and methodsStudy design and patient selectionThis was a retrospective cohort single center study. All allogeneic transplant procedures from June 2017 through September 2019 were included, with data collection until September 2020. Median follow-up was 17 months (interquartile range, IQR, 8.1–25.9 months).
Data collectionAll data were collected retrospectively from the patients’ medical records. Demographic and clinical data included: age, gender, significant past medical history of arterial hypertension, diabetes mellitus and CKD; basal kidney function characterization by estimated glomerular filtration rate (eGFR) using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for adults or Revised Schwartz equation for children/adolescents, measured GFR with EDTA test when available and proteinuria; previous hematological diagnosis/indication for HSCT; more than 3 previous lines of treatment; disease status pre-HSCT; donor characteristics, source and cryopreservation of hematopoietic progenitor cells; conditioning regimen; GvHD prophylaxis; days of hospitalization; occurrence of early complications post-HSCT (infection, use of antibiotics, antivirals and antifungals, acute GvHD, sinusoidal obstruction syndrome (SOS), TA-TMA, intensive care unit (ICU) admission, need for mechanical ventilation (MV); AKI in the first 100 days post-HSCT, its’ etiology and need for renal replacement therapy. We also documented the eGFR, proteinuria and albuminuria at 30 days, 100 days and one-year post-HSCT.
AKI is defined according to the KDIGO classification system11 as an abrupt decrease in kidney function occurring over 7 days or less. It can be further divided into 3 stages according to the severity of increase of baseline creatinine values. CKD is defined as a measured or estimated GFR <60ml/min/1.73m2, or the presence of markers of kidney damage (e.g., albuminuria) for >90 days.11
Since not all patients had GFR measured by EDTA, we chose to use eGFR calculated by CKD-EPI formula to allow comparison between groups.
Follow-up was considered until last observation or death during the data collection period.
Statistical analysisAssociation between each study variable and AKI was first explored using chi-square and Fisher's exact test for categorical variables. Univariate logistic regression model was used next to determine which variables could be of interest.
Survival curves for mortality were obtained with the Kaplan–Meier method and univariate Cox regression model was used to determine which variables could be of interest as predictors of long-term mortality.
Multivariate analysis was performed to determine the independent predictors of AKI, adjusting a logistic regression model, and to determine the predictors of mortality, adjusting a Cox regression model. All statistically significant variables in the univariate analysis and those considered clinically relevant were included.
Results are expressed as odds ratios (OR) for all logistic regression models and as hazard ratios (HR) for all Cox regression models, both with 95% confidence intervals (95%CI). A p-value of <0.05 was considered statistically significant. All analyses were performed using R statistical software (version 4.0.3).
ResultsPatient characteristicsDuring the study period, 155 patients were transplanted, whose main characteristics are presented in Table 1. Most patients were adults without significant comorbidities, and acute myeloid leukemia was the most common indication for HSCT.
Patients’ baseline characteristics.
| Patient characteristics | Children/adolescents | Adults |
|---|---|---|
| N (%) | 19 (12.3) | 136 (87.7) |
| Female gender, N (%) | 9 (47) | 65 (47) |
| Age (years) | ||
| Range | 0–15 | 19–66 |
| Mean±SD | 5.7±4.6 | 44.3±13.3 |
| Glomerular filtration rate (CKD-EPI) | ||
| Mean±SD (mL/min/1.73m2) | 188.4±65.8 | 105.9±19.8 |
| Glomerular filtration rate (EDTA) | ||
| Mean±SD (mL/min/1.73m2) | 143.1±62.4 | 98.6±28.6 |
| Previous diagnosis (N) | ||
| Primary immunodeficiency (5) | Acute myeloid leukemia (45) | |
| Acute lymphoblastic leukemia (4) | Acute lymphoblastic leukemia (20) | |
| Fanconi Anemia (4) | Myelodysplastic syndrome (17) | |
| Others (6) | Hodgkin lymphoma (10) | |
| Aplastic Anemia (8) | ||
| Primary myelofibrosis (8) | ||
| Non-Hodgkin lymphoma (6) | ||
| Others (26) | ||
| Evidence of complete response pre-transplant (with MRD negative, when appropriate), N (%) | 6 (31.6) | 66 (48.5) |
| Comorbidities, N (%) | ||
| Arterial hypertension | None | 9 (6.6) |
| Diabetes mellitus | None | 5 (3.7) |
| Chronic kidney disease | None | 2 (1.5) |
| Proteinuria | None | 9 (11.7) (missing 59) |
| More than 3 previous lines of treatment | None | 15 (11) |
CKD-EPI – Chronic Kidney Disease Epidemiology Collaboration; EDTA – ethylenediamine tetraacetic acid; MRD – measurable residual disease.
The characterization of the HSCT procedures and the main complications in the early post-HSCT period are presented in Table 2.
Transplant procedure characteristics and frequency of early complications.
| Transplant procedure characteristics | N (%) |
|---|---|
| Total | 155 (100) |
| Donor | |
| Matched related donor | 55 (35.5) |
| MUD | 98 (63.2) |
| Haploidentical | 2 (1.3) |
| Source of hematopoietic progenitors | |
| Peripheral blood | 130 (83.9) |
| Bone marrow | 23 (14.8) |
| Cord blood | 2 (1.3) |
| Cryopreserved hematopoietic progenitors | 17 (11) |
| Myeloablative conditioning | 82 (52.9) |
| GvHD prophylaxis | |
| Containing cyclosporine | 63 (40.6) |
| Containing tacrolimus | 90 (58.0) |
| Containing methotrexate | 67 (43.2) |
| Transplant procedure complications | |
| Infectious complications | |
| Febrile syndrome | 121 (78.1) |
| Viral reactivation (serum) | 49 (31.6) |
| Cytomegalovirus (CMV) | 42 |
| Adenovirus | 2 |
| BK polyomavirus | 2 |
| CMV+adenovirus | 1 |
| CMV+BK polyomavirus | 2 |
| Viral reactivation (urine) | 19 (12.3) |
| BK polyomavirus | 17 |
| Adenovirus | 1 |
| BK polyomavirus+adenovirus | 1 |
| Acute GvHD | |
| Global grade ≥II | 54 (34.8) |
| Sinusoidal obstruction syndrome | 9 (5.8) |
| Transplant-associated thrombotic microangiopathy | 6 (3.9) |
CMV – Cytomegalovirus; GvHD – graft-versus-host disease; MUD – matched unrelated donor.
More than 60% of the HSCT performed were from a matched unrelated donor (MUD), and peripheral blood was the most frequent graft source (83.9%). Only a minority of HSCT used cryopreserved hematopoietic stem cells (11%). The median length of hospital stay for the transplant procedure was 28 days (IQR 23–35), with a maximum of 175 days.
During the early post-transplant period, 121 (78.1%) patients had a documented febrile syndrome. The most frequent class of antibiotics used was beta-lactam antibiotics (n=116). Fifty-six patients (36%) received more than one antibiotic either sequentially or in combination. Antifungal therapy was used in 50 patients (32%), most frequently caspofungin (n=39) and liposomal amphotericin-B (n=25). Antiviral therapy was initiated in 45 patients (29%), 12 of which received more than one agent. Ganciclovir/Valganciclovir was the most used antiviral (n=39), followed by foscarnet (n=13).
Acute GvHD was observed in a total of 62 patients (40%), of which 54 had grade II or higher acute GvHD (41, 7 and 6 with grades II, III and IV, respectively). SOS and TA-TMA were relatively rare complications. Fourteen patients (9%) were admitted to ICU in the early post-transplant period.
Kidney evaluation in the post-HSCT periodRegarding kidney evaluation of our sample, we verified that the median eGFR was similar at 30 days, 100 days and one year after transplant: 99 (IQR 76–116), 100 (IQR 77–116) and 103 (IQR 85–115) ml/min/1.73m2 among adults, respectively, and 127 (IQR 106–165), 141 (IQR 121–192) and 118 (IQR 106–156) ml/min/1.73m2 among children/adolescents, respectively). At one-year post-HSCT, only two adult patients had CKD criteria.
Adult patients had a median proteinuria of 133mg/24h (IQR 77–367) and 123mg/24h (IQR 72–347) at 30 days and 100 days post-HSCT, respectively. Median albuminuria at these time points was 22.7mg/24h (IQR 11.4–107.2) and 11mg/24h (IQR 5.8–27.0), respectively. However, there were many missing data regarding evaluation of albuminuria and proteinuria (e.g. at 30 days, no value was observed in 140 patients (18 children and 122 adults). Therefore, these results must be interpreted with caution.
Acute kidney injury in the early post-HSCT settingAKI was observed in 50 patients (32%) during the first 100 days post-transplant. We identified 30 patients in KDIGO AKI stage I, 12 in stage II and 8 in stage III. Only six patients were evaluated (and oriented) by a Nephrologist, all of whom needed renal replacement therapy, either intermittent hemodialysis (n=1) or continuous venovenous hemodiafiltration (n=5). All six patients were admitted into the ICU, needed MV and died within the first three months post-transplant.
The etiology of AKI included drug nephrotoxicity, particularly from tacrolimus, valganciclovir, cidofovir and colistin (n=13), sepsis/septic shock (n=10), mainly pre-renal due to diarrhea, vomiting or mucositis (n=8), BK hemorrhagic cystitis (n=4), SOS (n=2), urinary tract infection (n=1) and TA-TMA (n=1). Unfortunately, we were not able to determine the cause of AKI in 11 patients.
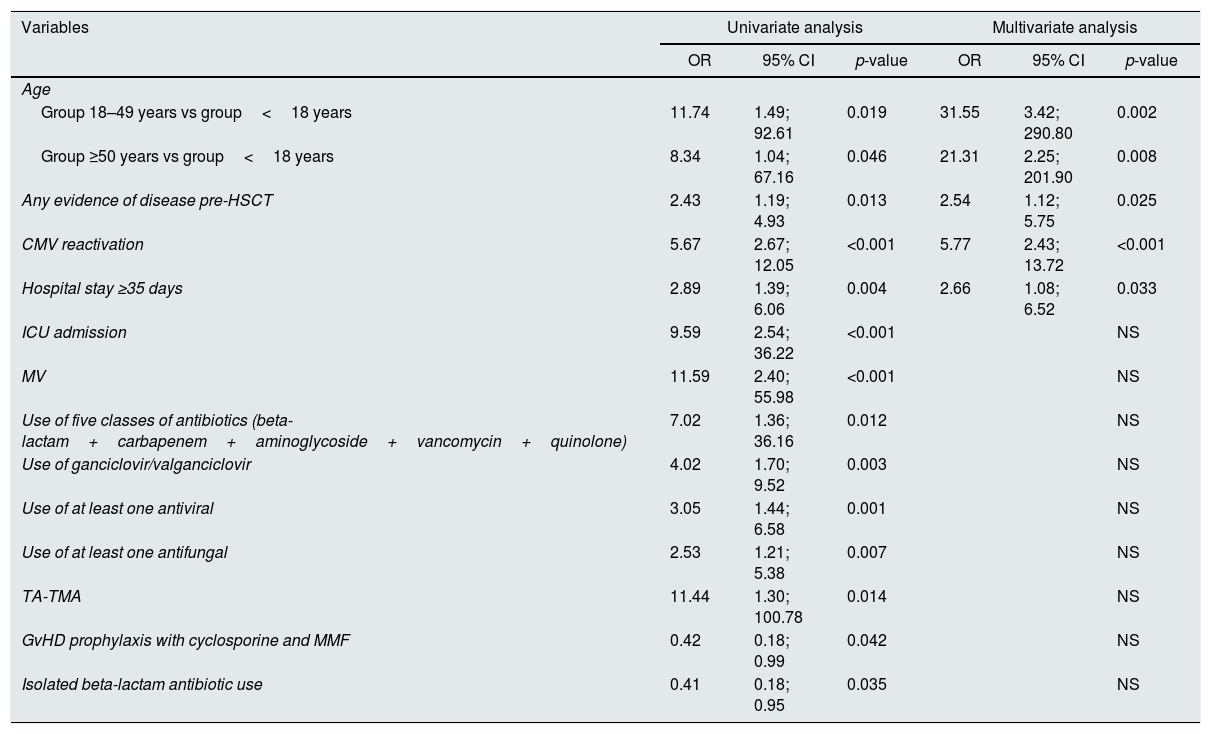
AKI predictors in the early post-HSCT settingVariables significantly associated with AKI in the early post-transplant period in univariate and multivariate analysis are discriminated in Table 3. In univariate analysis, in contrast to most variables studied, GvHD prophylaxis with cyclosporine and mycophenolate mofetil (as used in matched related donor RIC HSCT) and isolated beta-lactam antibiotic use were associated with a lower AKI risk.
Variables significantly associated with AKI in univariate and multivariate analysis.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | ||||||
| Group 18–49 years vs group<18 years | 11.74 | 1.49; 92.61 | 0.019 | 31.55 | 3.42; 290.80 | 0.002 |
| Group ≥50 years vs group<18 years | 8.34 | 1.04; 67.16 | 0.046 | 21.31 | 2.25; 201.90 | 0.008 |
| Any evidence of disease pre-HSCT | 2.43 | 1.19; 4.93 | 0.013 | 2.54 | 1.12; 5.75 | 0.025 |
| CMV reactivation | 5.67 | 2.67; 12.05 | <0.001 | 5.77 | 2.43; 13.72 | <0.001 |
| Hospital stay ≥35 days | 2.89 | 1.39; 6.06 | 0.004 | 2.66 | 1.08; 6.52 | 0.033 |
| ICU admission | 9.59 | 2.54; 36.22 | <0.001 | NS | ||
| MV | 11.59 | 2.40; 55.98 | <0.001 | NS | ||
| Use of five classes of antibiotics (beta-lactam+carbapenem+aminoglycoside+vancomycin+quinolone) | 7.02 | 1.36; 36.16 | 0.012 | NS | ||
| Use of ganciclovir/valganciclovir | 4.02 | 1.70; 9.52 | 0.003 | NS | ||
| Use of at least one antiviral | 3.05 | 1.44; 6.58 | 0.001 | NS | ||
| Use of at least one antifungal | 2.53 | 1.21; 5.38 | 0.007 | NS | ||
| TA-TMA | 11.44 | 1.30; 100.78 | 0.014 | NS | ||
| GvHD prophylaxis with cyclosporine and MMF | 0.42 | 0.18; 0.99 | 0.042 | NS | ||
| Isolated beta-lactam antibiotic use | 0.41 | 0.18; 0.95 | 0.035 | NS | ||
CMV – cytomegalovirus; GvHD – graft-versus-host disease; HSCT – hematopoietic stem cell transplant; ICU – intensive care unit; MMF – mycophenolate mofetil; MV – mechanical ventilation; NS – not significant; TA-TMA – transplant-associated thrombotic microangiopathy.
In multivariate analysis, only four variables remained as independent predictors for increased risk of AKI (Table 3): adult age (OR 31.55, 95%CI [3.42;290.8], p=0.002, and OR 21.31, 95%CI [2.25;201.9], p=0.008 for patients 18–49 years and ≥50 years, respectively), any evidence of disease pre-HSCT (OR 2.54, 95%CI [1.12;5.75], p=0.025), CMV reactivation (OR 5.77, 95%CI [2.43;13.72], p<0.001), and hospital stay longer than 35 days (OR 2.66, 95%CI [1.08;6.52], p=0.033).
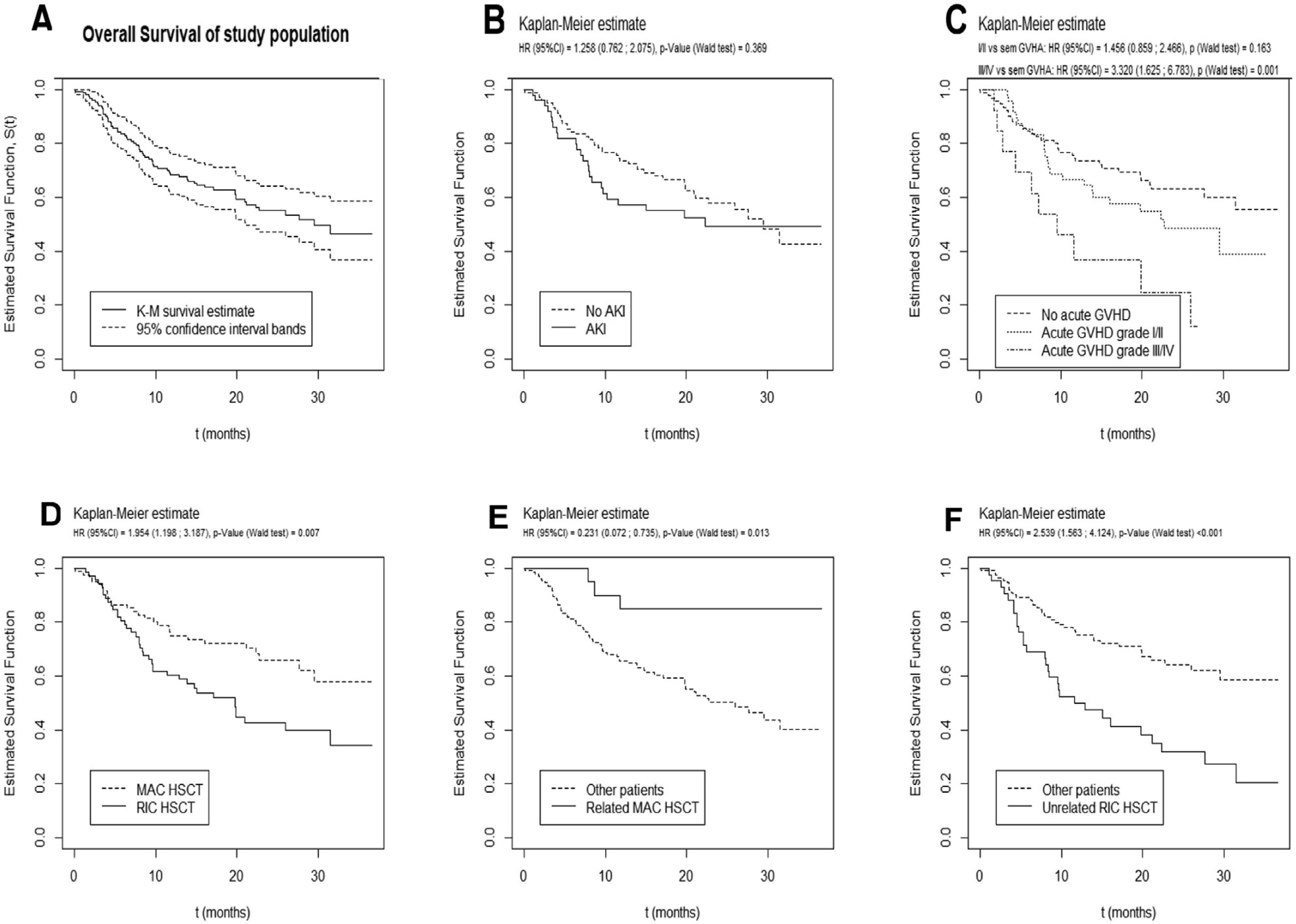
Prognosis and survival analysisWith a median follow-up time of 17 months (IQR 8.1–25.9 months), 67 patients died, 28 of which in the ICU. The median overall survival was 29.6 months (95%CI [21.2; not reached], Fig. 1).
Significant results of univariate and multivariate analysis for survival after allogeneic HSCT are described in Table 4. In univariate analysis, a higher maximum serum creatinine at 30 days post-HSCT and the need for renal replacement therapy correlated with worse survival. However, overall survival was not significantly different between patients with or without documented AKI, despite a trend toward a worse prognosis in the first year (Fig. 1B).
Variables significantly associated with survival after allogeneic HSCT in univariate and multivariate analysis.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | HR | 95% CI | p-value | |
| Patient characteristics | ||||||
| Age ≥50 years vs <50 years (continuous variable) | 1.66 | 1.03; 2.69 | 0.038 | 1.02 | 1.00; 1.04 | 0.029 |
| HSCT characteristics | ||||||
| Non-MAC vs MAC | 1.95 | 1.20; 3.19 | 0.007 | NS | ||
| Composite MAC/related donor | 0.23 | 0.07; 0.74 | 0.013 | NS | ||
| Composite RIC/unrelated donor | 2.54 | 1.56; 4.12 | <0.001 | 1.91 | 1.10; 3.33 | 0.022 |
| Hospital stay (continuous variable) | HR 1.02 | 1.01; 1.03 | 0.001 | 1.02 | 1.01; 1.03 | 0.002 |
| Complications post-HSCT | ||||||
| CMV reactivation | 2.38 | 1.43; 3.95 | 0.001 | NS | ||
| Any Acute GvHD | 1.74 | 1.07; 2.81 | 0.024 | NS | ||
| Acute GvHD Grade III/IV vs no GvHD or grades I/II | 2.87 | 1.46; 5.65 | 0.002 | 2.41 | 1.15; 5.03 | 0.019 |
| TA-TMA | 5.07 | 1.97; 13.0 | 0.001 | NS | ||
| ICU admission | 8.97 | 4.75;16.95 | <0.001 | NS | ||
| Mechanical ventilation | 8.59 | 4.34; 17.01 | <0.001 | 3.49 | 1.54; 7.92 | 0.003 |
| Antimicrobial therapies | ||||||
| Use of five classes of antibiotics (beta-lactam+carbapenem+aminoglycoside+vancomycin+quinolone) | 4.11 | 1.82; 9.23 | 0.001 | NS | ||
| Therapy with at least one antiviral | 2.30 | 1.42; 3.73 | 0.001 | NS | ||
| Maximum serum creatinine at 30 days post-HSCT (continuous variable) | HR 1.66 | 1.17; 2.37 | 0.005 | NS | ||
| Renal replacement therapy | 20.3 | 6.99; 58.85 | <0.001 | NS | ||
CKD – chronic kidney disease; GvHD – graft-versus-host disease; HSC – hematopoietic stem cells; ICU – intensive care unit; MAC – myeloablative conditioning; MUD – matched unrelated donor; RIC – reduced intensity conditioning; SOS – sinusoidal obstruction syndrome; TA-TMA – transplant-associated microangiopathy.
In multivariate analysis, only increasing age (HR 1.02, 95%CI [1.00;1.04], p=0.029), increasing length of hospital stay (HR 1.02, 95%CI [1.01;1.03], p=0.002), MUD RIC HSCT (HR 1.91, 95%CI [1.10;3.33], p=0.022), occurrence of grade III/IV acute GvHD (HR 2.41, 95%CI [1.15;5.03], p=0.019) and the need for MV (HR 3.49, 95%CI [1.54;7.92], p=0.003) predicted an inferior survival (Table 4). AKI from any etiology during the first 100 days post-transplant did not correlate with a worse survival.
DiscussionAllogeneic HSCT is a complex procedure that has been increasingly used as a curative approach for many malignant and non-malignant hematological diseases. The most frequent indications for HSCT in our population were in accordance with international data.3
AKI is a recognized complication in the HSCT setting and it may imply a worse prognosis.1 It is often multifactorial and is more common in allogeneic than in autologous HSCT.1,5,12,13 The frequency of AKI observed in this study (32%) is in the lower range of that reported in the literature.1,14,15 No significant differences were seen between RIC or MAC regimens, in accordance with previous results.1,5,6,16 AKI etiology observed in our sample is also in agreement with other studies.10
Even though AKI was observed in 50 patients, only six had a Nephrologist consultation, which correspond to patients who needed dialysis. The high mortality in the dialysis group is in line with that previously reported.7–10 Twenty patients had an AKI KDIGO stage 2 or more, and therefore could have benefited from specialized support. Only two patients had documented CKD at one-year post-HSCT, none of whom with previously documented AKI, and one with known CKD pre-HSCT. Therefore, it was not possible to demonstrate the influence of early AKI in CKD development in this study. This might have been limited by missing albuminuria and proteinuria data (more than 77%), and a short follow-up time for CKD development.
Another limitation of this analysis is the fact that it is mostly based on serum creatinine values. Since patients frequently lose weight and muscle mass during the peri-HSCT period and their hydration status is not steady over time, these results may be misleading. Using Cystatin C (not available at the time) might have allowed a more accurate analysis.
In this study, we aimed to identify predictors of AKI in the post-HSCT period. In univariate analysis, we observed that patients between 18 and 49 years had the highest risk for AKI, which could be explained by a greater exposure to aggressive treatments as compared to older adults.
Variables that indirectly relate to worse clinical condition, such as a longer hospital stay, ICU admission, the need for MV, and use of antifungal, antiviral or more complex antibiotic therapy, correlated with an increased risk of AKI, as previously described in the literature.10 CMV reactivation is a potentially serious complication associated with the compromised immune function in the post-HSCT period, and could cause target organ disease and multiorgan dysfunction, including AKI.14 Antiviral treatment directed at CMV is also potentially nephrotoxic. TA-TMA is a recognized complication of HSCT due to endothelial damage by chemotherapy, GvHD, infection, and use of calcineurin inhibitors.17 The kidney is one of the most affected organs in TA-TMA due to its rich microvasculature and high blood supply, so its association with AKI was expected.
The use of cyclosporine per se was not related to AKI, despite theoretical implications related to its effects in renal perfusion besides its contribution in TA-TMA risk. Other studies also failed to find this correlation.1,4,14 Cyclosporine plus mycophenolate mofetil are given as GvHD prophylaxis to the patients submitted to a matched related donor RIC HSCT. They are usually exposed to lower intensity drugs and have a lower risk of overall complications, which might help to explain why these patients have a smaller risk of AKI. Infectious complications that are treated with only a beta-lactam antibiotic (the first antibiotic empirically used when febrile complications arise) are more likely to have no major complications and a more rapid discharge from hospital, hence the correlation with a lower risk of AKI.
In multivariate analysis, only four of these variables independently predicted an increased risk of AKI during the early post-transplant period: adult age, any evidence of disease pre-HSCT, CMV viremia, and prolonged hospital stay. These may reflect an increased susceptibility to AKI, due to worse clinical condition and/or exposure to more potentially nephrotoxic drugs.
Regarding mortality predictors, we found that patients who had a RIC HSCT had a higher probability of worse survival than patients who received a MAC regimen, particularly if from a MUD. The nature of this study does not allow to clarify these results, but it is possible that this relates to older age, greater comorbidity load or greater risk of relapse of patients who receive a RIC, as opposed to a greater transplant-related mortality expected with a MAC regimen. Development of GvHD is more probable in MUD HSCT, which could also contribute to these findings. MUD RIC HSCT remained as an independent predictor of mortality in the multivariate model.
It is not unexpected that patients who experience serious early complications of HSCT, such as severe infection, TA-TMA or severe acute GvHD (indirectly represented by a longer hospital stay, more complex antibiotic regimens, ICU admission, need for organ support such as MV), have a worse outcome, as shown in the univariate analysis. CMV reactivation related to a worse survival, as expected.18 Increasing age at the time of HSCT also related to worse outcomes. In multivariate analysis, only age, length of hospital stay, the need for MV and grade III/IV acute GvHD remained as independent predictors of mortality, probably representing patients with more severe clinical condition.
Previous studies support that AKI correlates with a worse survival in the post-HSCT setting 15,18–20 and this could be more significant for more severe or earlier (pre-engraftment) AKI.7,15,16 Our results, however, did not support those findings. This could be due to the method used to determine the presence of AKI (serum creatinine), lack of albuminuria data, which is a recognized marker of kidney injury, a predictor of CKD and is associated with increased mortality,1 or relatively short follow-up time compared to other studies. AKI in allogeneic transplanted patients could be more frequently a consequence of an underlying acute condition such as GvHD or severe bacterial, fungal or viral infection, and therefore not an independent predictor of mortality. Dialysis requirement correlated with a high mortality, as described in the literature,7 but only in univariate analysis, perhaps due to its occurrence in only six patients.
This study has limitations, particularly due to its retrospective nature. Besides, it is sometimes difficult to determine the confounding or overlapping effect of some of the variables analyzed. Nevertheless, this study collects a relevant sample of HSCT procedures. Although the follow-up time is not very long, it allows for an analysis of early AKI development and related outcomes.
ConclusionsPatients submitted to HSCT are at an increased risk for AKI, not only due to exposure to many potentially nephrotoxic drugs, but also to the risk of complications such as sepsis, GvHD, CMV disease, SOS and TA-TMA, so they could benefit from specialized Nephrologist consultation as part of the multidisciplinary team. Although previous studies have reported decreased survival associated with AKI, we could not demonstrate a survival impact of AKI development in the early post-HSCT period. In the future, it would be interesting to evaluate prospectively the underlying causes, timing and impact of AKI development in the peri-HSCT period.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestNone declared.
We thank Adriana Belo for her contribution with the statistical analysis.