To investigate the efficacy of combined immunosuppressive regimens of cyclosporine (CsA), tacrolimus (TAC), or cyclophosphamide (CTX) combined with steroids in the treatment of idiopathic membranous nephropathy (IMN).
Materials and methodsA total of 150 biopsy-proven IMN patients were divided into three groups: CTX, TAC, and CsA groups (50 cases each). Patients received a selected regimen for 48 weeks. The efficacy (remission rate, 24h urinary protein, and serum albumin and creatinine) and safety (adverse events) profiles of administered regimens were evaluated at 12, 24 and 48 weeks.
ResultsAt 12 weeks, the response rates for CsA, TAC, and CTX groups were 14%, 50%, and 22%, respectively. This increased to 74%, 84%, and 82%, respectively at 48 weeks. During follow-up, 24h urinary protein significantly reduced from baseline in all regimens (P<0.05), while serum albumin increased in TAC and CTX groups after 12 weeks (P<0.05), and CsA group at 48 weeks (P<0.05). No significant changes in serum creatinine levels were noted in all three regimens (P>0.05). Safety was comparable in all groups, with lower respiratory tract infection being the most frequent adverse event.
ConclusionsThe combined regimens (i.e., TAC, CsA, and CTX) are effective in the treatment of patients with IMN at 48 weeks, while TAC and CTX might be more beneficial in terms of shortened time to remission and increased complete response rate.
Investigar la eficacia de los regímenes inmunosupresores combinados de ciclosporina (CsA), tacrolimús (TAC) o ciclofosfamida (CTX) combinados con esteroides en el tratamiento de la nefropatía membranosa idiopática (NMI).
Materiales y métodosUn total de 150 pacientes con NMI comprobada por biopsia se dividieron en 3 grupos: grupos CTX, TAC y CsA (50 casos cada uno). Los pacientes recibieron un régimen seleccionado durante 48 semanas. Se evaluaron los perfiles de eficacia (tasa de remisión, proteína en orina de 24h y albúmina y creatinina séricas) y seguridad (eventos adversos) de los regímenes administrados a las 12, 24 y 48 semanas.
ResultadosA las 12 semanas, las tasas de respuesta para los grupos CsA, TAC y CTX fueron del 14, el 50 y el 22%, respectivamente. Esto aumentó al 74, el 84 y el 82%, respectivamente, a las 48 semanas. Durante el seguimiento, la proteína urinaria de 24h se redujo significativamente desde el inicio en todos los regímenes (p <0,05), mientras que la albúmina sérica aumentó en los grupos TAC y CTX después de 12 semanas (p <0,05) y el grupo CsA a las 48 semanas (p <0,05). No se observaron cambios significativos en los niveles de creatinina sérica en los 3 regímenes (p> 0.05). La seguridad fue comparable en todos los grupos, siendo la infección del tracto respiratorio inferior el evento adverso más frecuente.
ConclusionesLos regímenes combinados (es decir, TAC, CsA y CTX) son eficaces en el tratamiento de pacientes con NMI a las 48 semanas, mientras que TAC y CTX podrían ser más beneficiosos en términos de reducción del tiempo de remisión y aumento de la tasa de respuesta completa.
Idiopathic membranous nephropathy (IMN) is characterized by the presence of proteinuria and incrassation of the glomerular basement membrane with spike-like pathologic changes. The incidence of IMN is considerably high in adults, with gender predominance in males. While the pathogenesis of IMN is not clearly understood, immunofluorescence (IF) has revealed that granulated complement C3 and IgG gradually accumulate along the glomerular capillary wall (GCW) in the kidneys of diseased patients. Moreover, phospholipase A2 receptor (PLA2R), present in glomerular podocytes, and thrombospondin type-1 domain-containing 7A (THSD7A) act as antigens, when bound to antibodies (mainly IgG4), they lead to the activation of complements in 70-80% of cases. This may result in the in situ deposition of circulating immunocomplexes under neath glomerular epithelial cells.1,2 These events are peculiar to IMN and are rare in secondary membranous nephropathy, therefore, IMN has been considered as an autoimmune disease.
Despite treatment, patients with IMN could have either worsening renal function (WRF) or undesired release. Regardless of immunosuppressive therapy, Hogan et al.3 reported that the 5-, 10-, and 15-year survival rates in 1189 cases with IMN were 86%, 65%, and 59%, respectively. Furthermore, 40% of patients ended up with end-stage renal disease (ESRD). Meanwhile, Aaltonen et al.4 noted spontaneous remission (mostly within 12 months after diagnosis) in 13/76 cases with IMN, among whom five cases had recurrent disease later Meanwhile, others had spontaneous remission after four years. Chen Y et al.5 indicated that 32% of IMN patients who had not received any treatment could achieve spontaneous remission and have a favorable prognosis, of which only 5.7% were recurrent afterward. While most patients usually have stable renal functions, some patients may advance to renal failure with poor prognosis. Therefore, there is a possibility of either spontaneous remission or end-stage renal disease with impaired renal function due to the slow progression process of IMN.
Currently, cytotoxic drugs such as cyclophosphamide (CTX), chlorambucil (CH) and cyclosporine A (CsA) are widely used in the treatment of IMN. In the Clinical Practice Guidelines of KDIGO,6 CTX in combination with a glucocorticoid is recommended as a prioritized regimen to treat IMN. Other agents have also been used, such as tacrolimus (TAC) and calcineurin inhibitor (CNI, such as cyclosporine). To date, the choice of the therapeutic regimen has not been optimized and standardized with no clear guidelines to support treatment decisions. Therefore, we conducted this investigation to compare the efficacy and safety of the administration of TAC, CsA, and CTX in combination with steroids for successive 48 weeks for the treatment of IMN.
Materials and methodsPatientsFrom January 2014 to July 2016, the medical records of patients with biopsy-proven IMN in our hospital were reviewed. Included participants were 20–75 years of age with a primary diagnosis of a pathological type I–III of IMN, PLA2R- positive by immunofluorescence, and IgG4 as the dominant IgG (C1q-negative). Additional criteria included serum albumin<30g/L and 24h proteinuria (after 6 months of conservative treatment or serious symptoms of nephropathy)>3.5g; serum creatinine (Scr)<309μmol/L. Patients with a history of organ transplant, secondary MN (i.e. hepatitis B and systemic lupus), coexistent renal diseases, diabetes mellitus, compromised immunity (i.e. HIV and cancer), or serious complications (i.e. severe infection) were excluded (Appendix 1). This study was conducted in accordance with the declarations of Helsinki's guidelines.
TreatmentsPatients received either CTX, TAC or CsAin conjunction with low-dose steroids (prednisone was initially given at a dose of lmg/kg/day, orally at 8 o’clock every morning after breakfast). After 8 weeks, it was gradually reduced by 10% every 2 weeks until a final dose of 0.15mg/kg/day. This dose was continued for a total of at least 48 weeks.
In the CTX group (50 cases), patients received CTX as an intravenous infusion twice a month, within a dose range of not more than 1g/month. Actually, the therapeutic dose of CTX for patients weighing less than 33.33kg is 15mg/kg, while patients weighing more than 33.33kg is 500mg each time.
In the TAC group (50 cases), patients were treated with oral TAC every 12h, with the daily dose limited within a range of 0.05–0.075mg/kg, for six months. The drug levels in blood were measured after one week and then once per month, with the Cmin maintained at 5–10μg/L. In the CsA group (50 cases), patients were given CsA orally every 12h, with a daily dose of no more than 3.5–5.0mg/kg for six months. The drug levels in blood were measured after one week, followed by once per month. Once the concentration of 333–500nmol/L (2h after administration)was achieved, the Cmin was maintained at 104–146nmol/L later on. After treatment for 6 months, the doses of all drugs were gradually reduced once the patient achieved clinical remission (CR) or partial response (PR), and then the same dose was maintained from at least 48 weeks to 18 months.
During treatment, liver functions and blood glucose levels were assessed and liver-protective drugs and hypoglycemic agents were prescribed if needed, respectively. In addition, since CTX was given by intermittent intravenous infusion in this study, all recruits accepted treatments in accordance with the established scheme, and there were no cases of reduced use finally.
Dose reduction and discontinuationDuring treatment, the doses of assessed regimens were reduced for 14 days if patients had symptoms of renal insufficiency (i.e. significant reduction in urine volume and creatinine increase by >30%). Once renal functions were recovered, drugs were increased back to the regular dose. However, if renal functions failed to improve, the drug was discontinued immediately.
Assessments and outcome measuresThe efficacy and adverse events (AEs) of assessed regimens were determined after treatment for 12, 24, and 48 weeks. The primary outcome was clinical remission (CR and RR) rates, which was determined by the improvement of renal functions (24h urine protein, serum albumin and creatinine, and estimated glomerular filtration rate (GFR)).
The efficacy of investigated regimens in terms of CR, PR, no response (NR), and recurrence11 was determined by two independent assessments with an interval of >7 days. CR was defined as 24h urine protein<0.3g/day (uPCR<30mg/mmol or <300mg/g), serum albumin>baseline level, and normal serum creatinine level. PR was defined as 24h urine protein<3.5g/day (uPCR<350mg/mmol or <350mg/g) with a decrease by 50% when compared to the peak level, serum albumin>baseline level, serum creatinine within normal or below the baseline level. NR was defined as the failure to reach the aforementioned criteria for CR and PR.
The secondary outcomes included safety, which was assessed by monitoring the effect on liver functions (transaminase and bilirubin) or other AEs (i.e. infection and new onset diabetes mellitus, etc.). Recurrence was defined as the reoccurrence of proteinuria (within range of renal diseases) after successful CR or PR.
Statistical analysisAll statistical analyses were performed using the SPSS 23.0 software. Student's t-test and one-way ANOVA were used for comparison between different groups. The descriptive data were expressed as frequency and percentage. Chi-square test and Fisher's exact test were used to compare multiple variables among different groups. Mean and standard deviations (SD) were used in continuous variables. A P-value of <0.05 was considered statistically significant.
ResultsClinical characteristicsThe retrospective recruitment of patients is presented in a flowchart (Fig. 1). A total of 150 patients were included in the final analysis (50 patients in each arm), 91 males and 59 females with a mean age of 47.7±11.6 years. All groups were well-balanced with respect to baseline demographic (age & gender) and clinical characteristics (24h urine protein, serum albumin, and creatinine, renal pathological staging (numbers of glomerular sclerosis, puncture glomerulus, segmental sclerosis, renal tubular, interstitial and vascular lesions)) (Tables 1 and 2).
Baseline demography and clinical characteristics of IMN patients.
| TAC | CSA | CTX | P value | |
|---|---|---|---|---|
| Gender (male/female) | 33/17 | 26/24 | 32/18 | 0.697 |
| Age (year) | 46.8±12.5 | 46.2±13.5 | 50.1±9.2 | 0.214 |
| 24h urine protein (g/d) | 7.61±1.60 | 6.37±1.83 | 7.03±1.41 | 0.119 |
| Serum albumin (g/L) | 21.91±5.36 | 23.79±4.27 | 22.02±3.55 | 0.968 |
| Serum creatinine (μmol/L) | 78.1±36.2 | 61.3±19.6 | 77.2±44.2 | 0.091 |
TAC: tacrolimus; CSA: cyclosporine; CTX: cyclophosphamide. Note: Values are expressed as mean±standard deviation unless specified otherwise.
Baseline renal pathology of IMN patients in three intervention groups.
| Renal pathology | TAC | CSA | CTX | P value |
|---|---|---|---|---|
| Pathological stages (I/II/III), n | 6/38/6 | 6/36/8 | 5/37/8 | 0.875 |
| Number of puncture glomerulus, mean±SD | 14.3±7.3 | 14.5±6.8 | 14.5±4.1 | 0.976 |
| Glomerular sclerosis, n (%) | 17 (34.0%) | 20 (40%) | 20 (40%) | 0.817 |
| Segmental sclerosis, n (%) | 8 (16.0%) | 7 (14.0%) | 8 (16.0%) | 0.923 |
| Renal tubular lesions, n (%) | 30(60.0%) | 23(46.0%) | 11 (55.0%) | 0.698 |
| Renal interstitial lesions, n (%) | 27(54.0%) | 30 (60.0%) | 25(50.0%) | 0.894 |
| Renal vascular lesions, n (%) | 25 (50.0%) | 23 (46.0%) | 27 (54.0%) | 0.872 |
SD: standard deviation; N: number; TAC: tacrolimus; CSA: cyclosporine; CTX: cyclophosphamide.
The efficacy of various regimens is presented in Table 3. At 12 weeks, 7 patients in the CsA group achieved PR but none achieved CR, with a remission rate of 14%. On the other hand, 3 patients in the TAC group achieved CR and 22 achieved PR, with are mission rate of 50%, while 3 cases in the CTX group achieved CR and 8 cases achieved PR, with a remission rate of 22%. In summary, the majority of patients who received CsA or CTX (≥70%) failed to respond to treatment, while 50%of patients responded to TAC at 12 weeks.
Comparison of efficacy among three intervention groups.
| Study group | Total | CR (n) | PR (n) | NR (n) | Rate of CR+PR (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | 12w | 24w | 48w | 12w | 24w | 48w | 12w | 24w | 48w | 12w | 24w | 48w | |
| CsA | 50 | 0 | 0 | 7 | 7 | 17 | 30 | 43 | 33 | 13 | 14% | 34% | 74% |
| TAC | 50 | 3 | 6 | 11 | 22 | 31 | 31 | 25 | 13 | 8 | 50% | 74% | 84% |
| CTX | 50 | 3 | 8 | 13 | 8 | 23 | 28 | 39 | 19 | 9 | 22% | 62% | 82% |
TAC: tacrolimus; CSA: cyclosporine; CTX: cyclophosphamide; CR: complete response; NR: no response; PR: partial response; W: week.
At 24 weeks, 37 patients who received TAC achieved PR (31cases) and CR (6 cases), with a remission rate of 74%, while in the CTX group, the number of patients who achieved PR and CR increased to 23 and 8 cases, respectively, resulting in a remission rate of 62% (Table 3). However, the remission rate in the CsA group increased only to 34% (17 achieved PR and none achieved CR), remaining the lowest among the three groups.
At 48 weeks, the remission rates for all 3 interventional groups were almost similar (74%, 84%, and 82% for CsA, TAC, and CTX, respectively. Meanwhile, the best response (CR) rates for CTX, TAC, and CsA were 26% (13/50), 22% (11/50), and 14% (7/50), respectively (Table 1).
In summary, patients in the TAC group had higher remission rates compared to CsA group at 12 and 24 weeks (50% vs 14% and 74% vs 34%; P<0.001), respectively. On the other hand, no significant differences between TAC and CTX or CTX and CsA groups were observed (P>0.05). Noteworthy, the differences between TAC and CsA were insignificant at 48 weeks (P>0.05), as all treatment regimens reached their peak levels (70–85%).
Improvement of renal functionWe estimated serum albumin and 24-h urinary protein levels at baselines, 12, 24, 36, and 48 weeks after treatment to determine the efficacy of each regimen at different time-points.
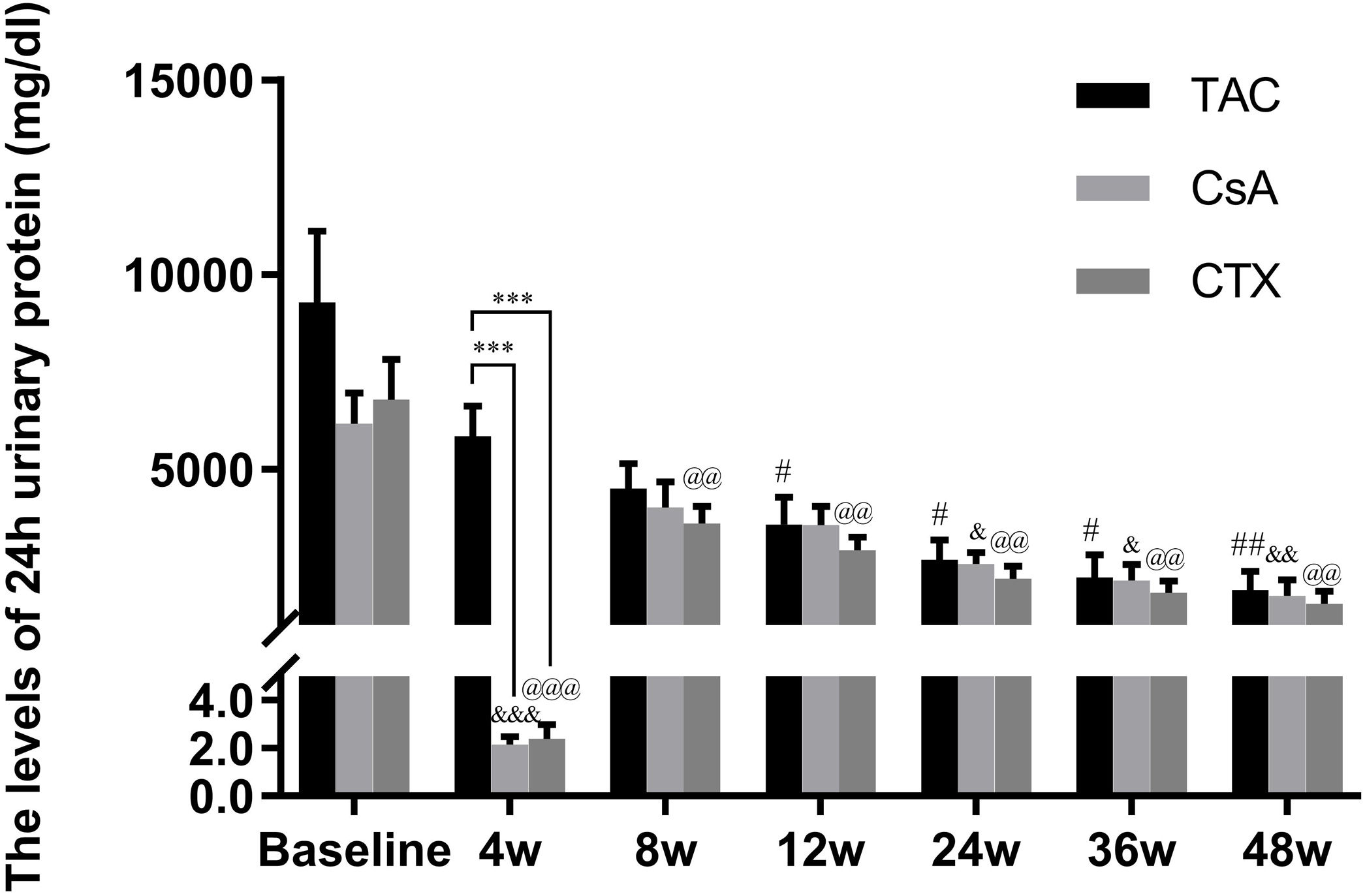
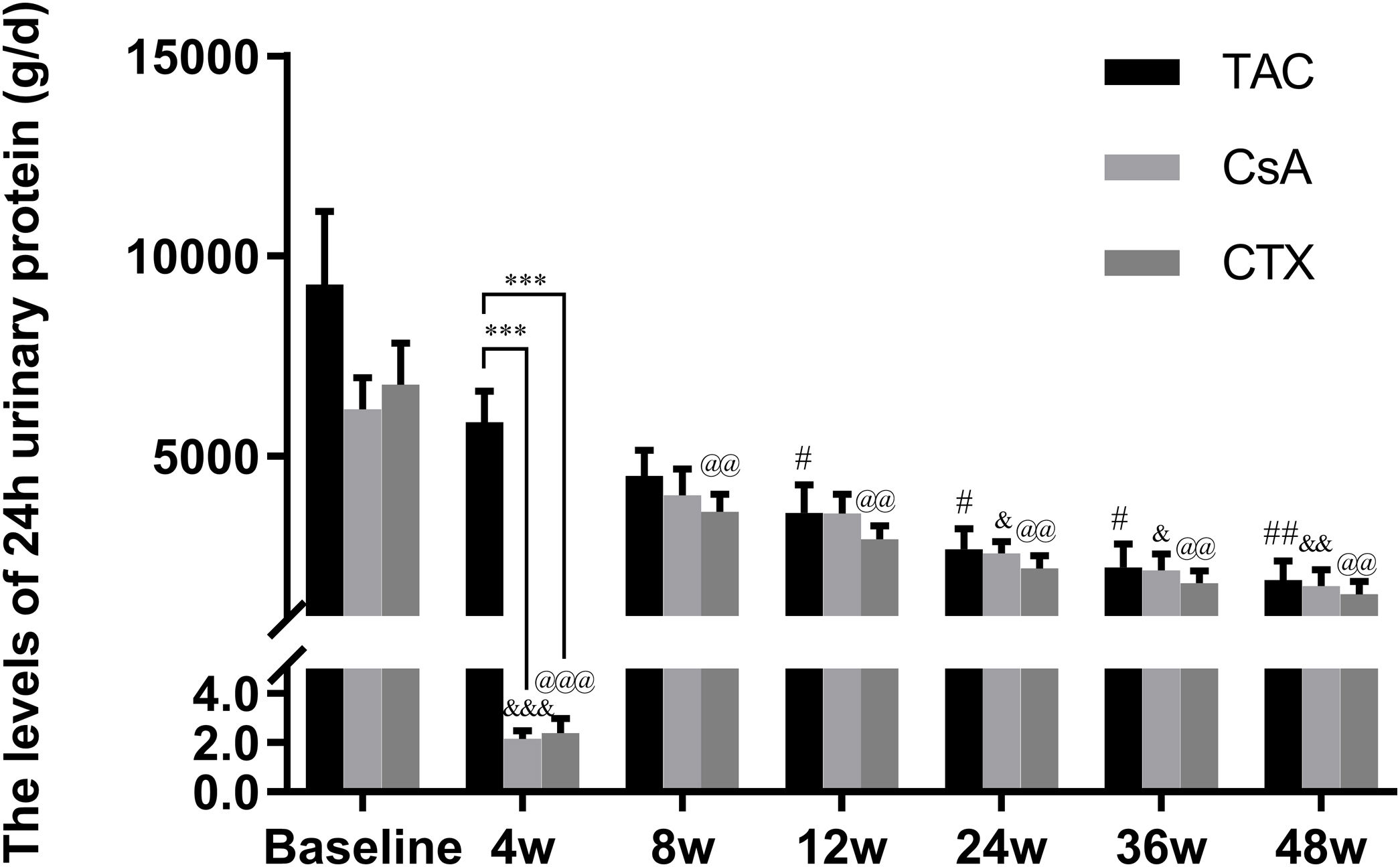
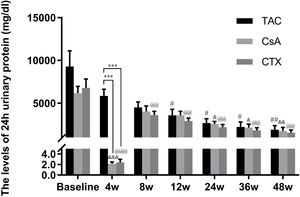
Baseline 24-hour urine protein levels in the TAC, CsA, and CTX groups were 7.61, 6.37, and 7.03g/d, respectively. At 12 weeks, TAC resulted in the highest reduction in 24-h urine protein levels from baseline (Mean percent of reduction=56.10%; P<0.01). At 24 weeks, TAC was also superior to other regimens in terms of 24-h urine levels, with a mean percent of reduction of 62.41 (P<0.01). At 36 and 48 weeks, TAC remained superior to other regimens with a mean percent of reduction of 69.67 (P<0.01) and 74.11 (P<0.01), respectively (Fig. 2).
Comparison of the 24h urinary protein levels before and after treatment among three intervention groups. The significant difference was found in 4 weeks post-treatment among three groups. The levels of 24h urinary protein continued to decrease and reached a platform at 24 weeks, when the reduction was significant in the group of TAC, CsA and CTX respectively. *P<0.05, **P<0.01 and ***P<0.001 compared with specific group. #P<0.05, ##P<0.01 and ###P<0.001 compared with the baseline of TAC group. &P<0.05, &&P<0.01 and &&&P<0.001 compared with the baseline of CsA group. @P<0.05, @@P<0.01 and @@@P<0.001 compared with the baseline of CTX group.
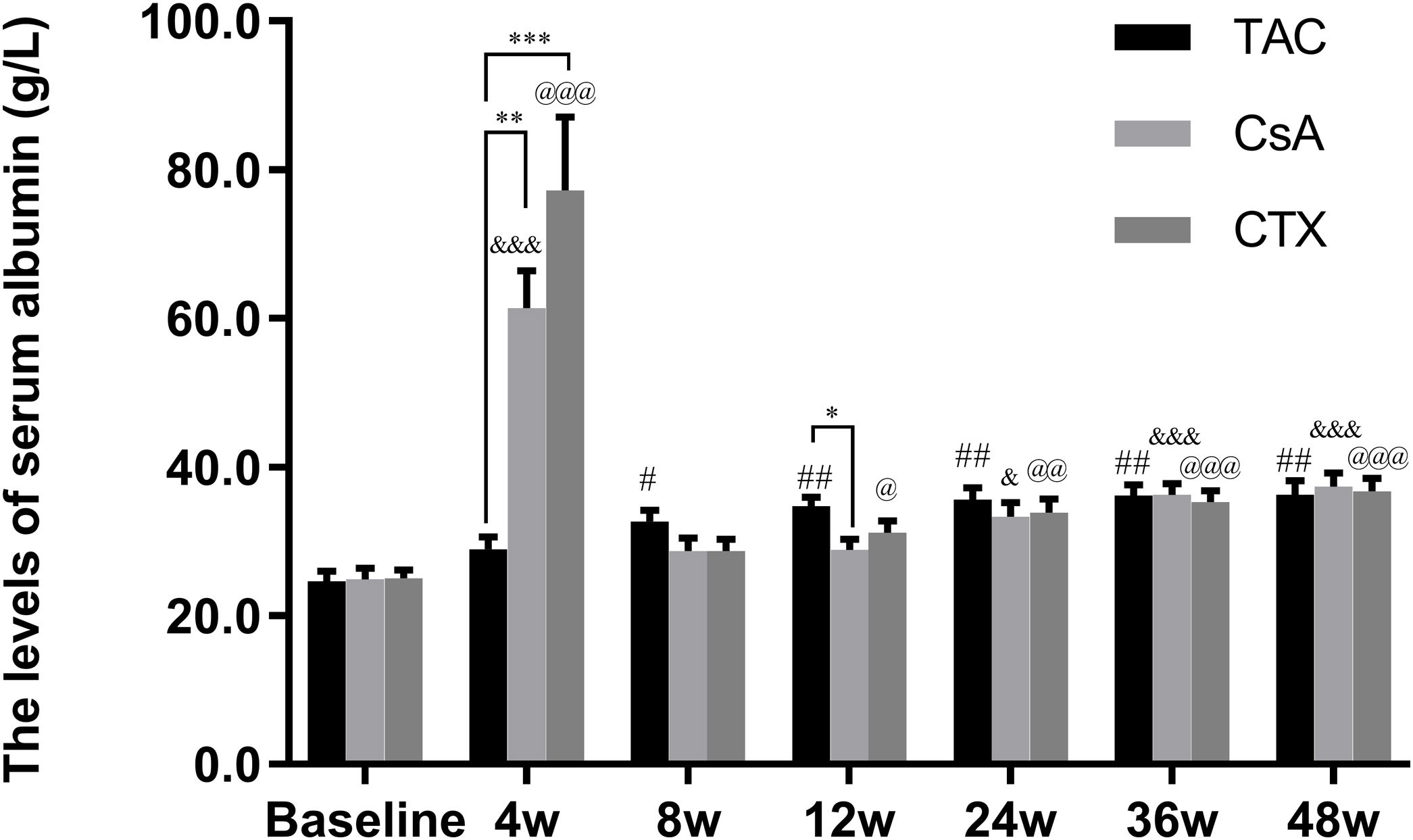
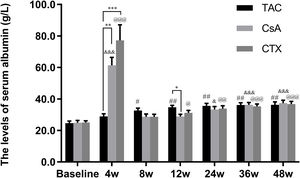
Furthermore, the baseline serum albumin levels in the TAC, CsA, and CTX groups were 21.91, 23.79, 22.02g/L, respectively. At 12, 24, and 36 weeks, TAC revealed superiority over the other two regimens in improving serum albumin levels with mean percent of increase of 49.27 (P<0.01), 52.04 (P<0.01), and 54.45 (P<0.01), respectively. In addition, at 48 weeks, CTX became superior to the other two drugs, resulting in the highest increase in serum albumin levels (Mean percent of increase=55.69; P<0.01) (Fig. 3).
Comparison of the serum albumin levels before and after treatment among three intervention groups. The effect of TAC on serum albumin levels was stable and sustained from 8 to 48 weeks post-treatment. After 24 weeks post-treatment, there was no significant difference among three intervention groups. *P<0.05, **P<0.01 and ***P<0.001 compared with specific group. #P<0.05, ##P<0.01 and ###P<0.001 compared with the baseline of TAC group. &P<0.05, &&P<0.01 and &&&P<0.001 compared with the baseline of CsA group. @P<0.05, @@P<0.01 and @@@P<0.001 compared with the baseline of CTX group.
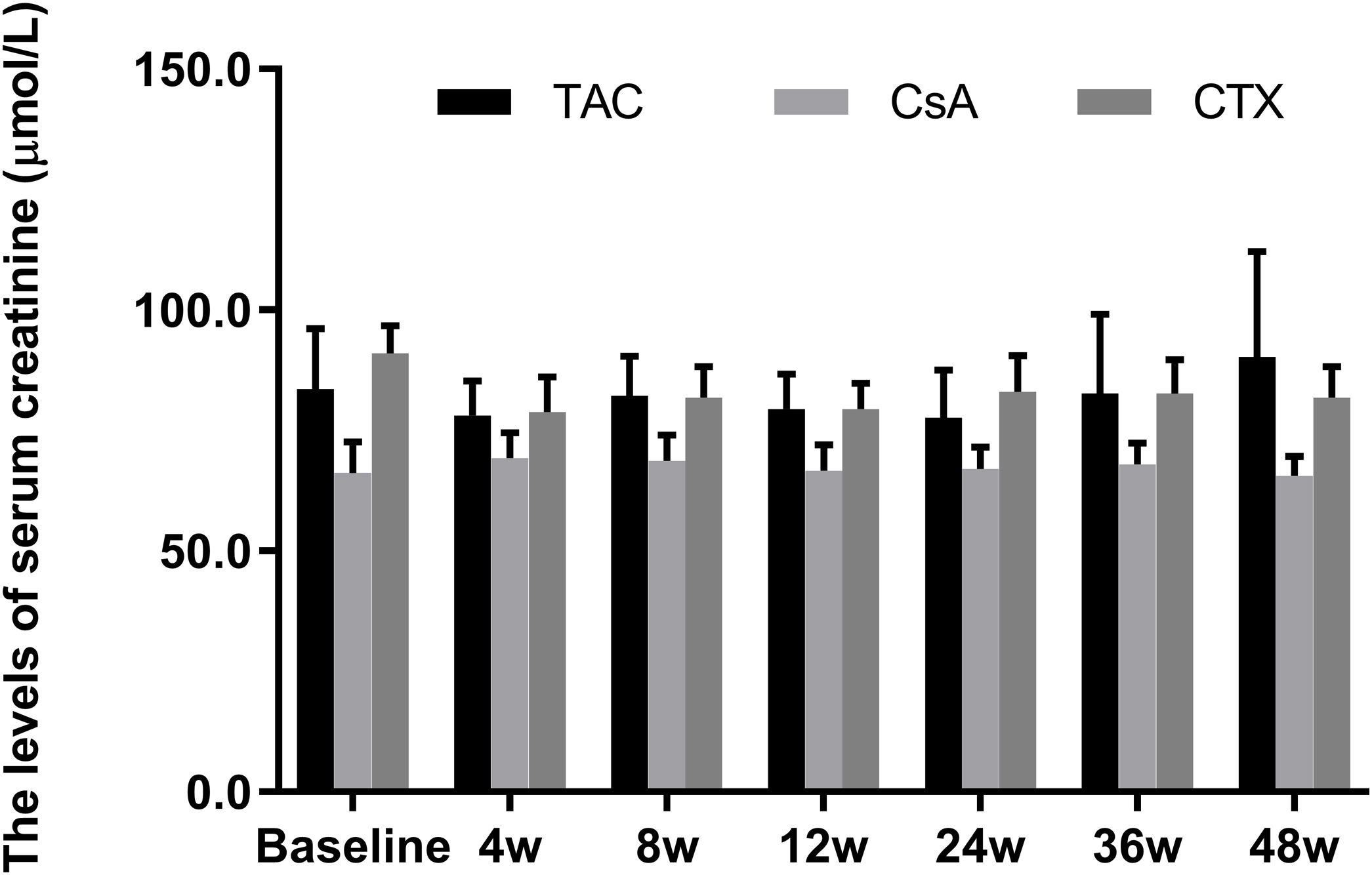
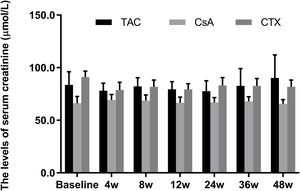
Meanwhile, the changes in serum creatinine levels from baselines at 12, 24, 36, and 48 weeks were not statistically significant in all three regimens (P-value>0.05) (Fig. 4).
Comparison of the serum creatinine levels before and after treatment among three intervention groups. There were no significant difference in serum creatinine levels from baselines at 4, 8, 12, 24, 36, and 48 weeks in all three regimens. *P<0.05, **P<0.01 and ***P<0.001 compared with specific group. #P<0.05, ##P<0.01 and ###P<0.001 compared with the baseline of TAC group. &P<0.05, &&P<0.01 and &&&P<0.001 compared with the baseline of CsA group. @P<0.05, @@P<0.01 and @@@P<0.001 compared with the baseline of CTX group.
Overall, all three regimens were well tolerated in IMN patients; however, only low-grade adverse events were observed in a few cases. During treatment, 2 patients in the CsA group, 1 patient in the CTX group, and 1 patient in the TAC group suffered from lower respiratory tract infection (LRTI). Meanwhile, one patient in the CTX group developed pulmonary embolism during treatment, while one casein the CsA group had a recurrence after achieved remission. Treatment was temporarily discontinued in one patient in the TAC group due to a sharp increase in serum creatinine level until the serum creatinine level went back to normal.
DiscussionPatients with membranous nephropathy (MN) are diagnosed as IMN upon the exclusion of all MN-causing factors. It is noteworthy that there are several break through in understanding the pathogenesis of IMN. In 2009, Jr BL et al.7 discovered M-type phospholipase A2 receptor (PLA2R) and anti-PLA2R antibodies, representing a milestone in understanding IMN pathogenesis. WhilePLA2R is weakly expressed in normal human glomerular podocytes, its expression is significantly increased in most glomerular podocytes of PLA2R-associated IMN patients, suggesting that enhanced glomerular PLA2R antigen staining is a more direct and sensitive biomarker for the diagnosis of PLA2R-associated IMN. Anti-PLA2R antibodies are mainly IgG4, which co-localize with PLA2R antigen in glomerular capillary loops, relying on conformational epitope. Later, several studies have revealed that anti-PLA2R antibodies display dynamic changes in titer, which correlates with the prognosis of IMN patients and therefore can be used as a marker to monitor disease status, therapeutic response, and recurrence.8
The present study targeted a major subset of patients with PLA2R-positive IMN, in whom IgG4 was the main type of IgG (C1q negative). The results indicated that IMN patients responded rapidly to TAC, followed by CTX, whereas the slowest response was observed in the CsA group. The analysis also revealed that the overall remission rates of patients treated with these 3 regimens were similar after 48 weeks of intervention. Noteworthy, patients with IMN might benefit more from CTX or TAC than CsA, as more patients achieved CR after being treated with TAC and CTX. Treatment with either TAC, CTX, and CsA resulted in significant improvement in the kidney functions of IMN patients, which is reflected by a marked reduction in 24-h urinary protein levels and marked elevation of serum albumin levels at 48 weeks compared to baseline data. This improvement of 24-h urinary protein levels and serum albumin levels was pronounced more in the TAC and CTX groups at 48 weeks, respectively.
Our observations suggest that the efficacy of the3 regimens, with respect to improvement of renal function, is very similar at the end of the 48th week, while the action of CsA seemed a bit slower than TAC and CTX in elevating serum albumin. Overall, all three interventional regimens were effective against IMN, manifested by a drastic increase in remission rates along with a marked improvement of renal function in addition to being well-tolerated.
The decision of treatment is usually made based on the level of urinary protein in MN patient, while patients with a relatively slight increase in urinary protein often do not need special treatment and can be treated only with angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB), or anti-platelet adhesives agents to promote the recovery of renal function.9 Immunosuppressive agents and steroids are generally not necessary for the treatment of these patients.10 However, after a standard conservative treatment with ACEI or ARB for 6 months, if 24h urinary protein continues to increase (>4g), while patients are prone to hypoproteinemia and hyperlipidemia, along with the deterioration of renal function, they should receive immunosuppressive therapy in order to reduce urinary protein, lower lipid, and prevent coagulation.11
Indeed, the findings of the present study highlight the outcomes of renal improvement following the administration of TAC, CTX, or CsA inpatients who failed to respond to conservative therapy for 6 months (worse renal functions), and how they could notably alleviate the patients’ condition. Even though the patients were well tolerant of these regimens, they should be closely monitored for some potential AEs such as infection (e.g., LRTI) and liver dysfunction.
Immunosuppressive therapy may increase the remission rate of patients with IMN.12 Despite that early treatment with immunosuppressive agents could slow down or delay the progression of nephritis, the long-term use of immunosuppressive agents might cause AEs, which in turn may worsen the prognosis of these cases. In a 20-year retrospective analysis, Sato M et al.13 demonstrated that the efficacy of steroids alone to treat IMN was no better than the standard supportive treatment. In fact, they might lead to more side effects and quicker deterioration of renal function. These findings suggest that other immunosuppressive agents combined with steroids might be needed to treat IMN.
Cytotoxic drugs, such as CTX and CsA, are widely researched for the treatment of IMN. Ponticelli et al.14 compared the efficacy of Methylprednisone (MP)+Chlorambucil (CH) and MP+CTX regimens by monitoring proteinuria. After three years of follow-up, no significant difference in the remission rate (PR and CR) was found between both groups (82% vs 93%). However, it is noteworthy that the drop-off rate of patients who failed to complete the treatment plan reached 14% in the MP+CH group. Meanwhile, Wu et al.15 adopted the modified Ponticelli regimen combining CTX with steroids to treat IMN in a Chinese cohort. They found that this therapy could relieve proteinuria in IMN patients; however, its long-term benefit remains to be defined. On the other hand, Zhou et al.16 reported that monotherapy of calcineurin inhibitor CsA exhibited a short-term effect but failed to improve the CR rate.
Furthermore, Usui et al.17 has retrospectively analyzed 32 IMN patients who were treated with CsA in a combination of low-dose steroids. It was found that while the patients achieved a satisfactory response, 50% of patients had recurrence after a follow-up period from 37.5 to 89.2 months. Ramachandran et al.18 investigated a combination of another calcineurin inhibitor TAC and steroids in comparison with the modified Ponticelli regimen. They found that the short-term remission rate in the TAC group was higher than that in the CTX group. Meanwhile, caution should be taken in the long-term use of TAC due to its potential side effects, such as amenorrhea as well as hepatic and renal toxicity. Even though several combined regimens have been reported in the literature, it remains to be determined which combined therapy is better. In the present study, the efficacies of the three regimens were comparable in terms of both renal function improvement and overall remission rate (PR+CR). The data also suggests that patients treated with CTX or TAC might gain more benefits, as they seem to have an earlier response (after 12-week treatment) with a higher probability to achieve CR than those treated with CsA.
That being said, careful consideration of the adverse effects of such regimens should be taken. In our study, one patient in the CTX group suffered from pulmonary embolism. Morbidity of MN-related pulmonary embolism and renal venous thrombosis could reach 11% and 35%, respectively.19 Thus, it is re-emphasized that close attention should be paid to monitor the coagulation activity during the treatment of IMN. In a single patient receiving TAC regimen, the intervention was temporarily discontinued due to a sharp increase in serum creatinine, however, serum creatinine level went back to the normal afterward.
Besides the insights of our findings into the treatment approach in cases with IMN, our study had several limitations. First, the small sample size of our study limited the power to identify small differences among investigated regimens. Second, we did not include patients with PLA2R-negative but THSD7A-positive IMN as THSD7A staining was not yet routinely used for the diagnosis of IMN in our clinic, and thus, this may restrict the generalization of our findings. Finally, the retrospective design of our study limits the generalizability of our findings secondary to the potential biases incorporated in our design. Therefore, further investigations for optimizing the selection of immunosuppressive agents or regimen type and determining the appropriate time to initiate therapy as well as the maintenance duration of treatment are warranted in order to make an individualized treatment plan for patients with IMN.
ConclusionsAll three immunosuppressive regimens (TAC, CTX, and CsA) in combination with steroids are effective and well-tolerable in the treatment of patients with PLA2R-positive IMN.TAC shows better short-term efficacy (12 weeks) than CTX (24 weeks) and CsA (48 weeks). More patients achieve CR after a48-week treatment with CTX (25%) and TAC (22%) than CsA (13%).
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.
We would like to thank the technical support from Dr. Weiyuan Lin. We are grateful for the time and efforts of the nephrologists who supported the present study: Dr. Yulin Zhang, Dr. Zili Zheng, Dr. Yun Zhang, Dr. Guangjian Liu, Dr. Quanlin Zheng, Dr. Yanqian Wang, Dr. Mengmeng Hong, and Dr. Chunhong Chen.