Arterial stiffness is nowadays a well-accepted predictor of cardiovascular mortality in general population; as well as in kidney transplant recipient population. The femoral-carotid pulse wave velocity (cf-PWV) is the widest used method to assess the arterial stiffness. The aim of this study was to test whether CNI-free immunosuppression based on belatacept was associated with lower cf-PWV, as a surrogate marker of arterial stiffness, than CNI. This was a retrospective case-control study. We included all the cases treated with belatacept as a maintenance immunosuppression in our center (n=20). An appropriate control group of patients (n=20) treated with CNI was selected to achieve match for key factors associated with arterial stiffness. After a follow-up of 5 years after transplantation, the Belatacept group had a reduced prevalence of patients with a cf-PWV higher than 8.1m/s (50% in BLC vs. 25% in CNI, p=0.08). At multivariate logistic regression analysis, the risk of high cf-PWV was correlated with age (OR 1.24; p<0.03) and renal resistive index (OR 1.25; p<0.05). Belatacept treatment was associated with a significant reduction in risk of cf-PWV (OR 0.008; P=0.045). Belatacept-based maintenance immunosuppression could improve kidney transplant recipient’s survival by reducing cardiovascular events related to stiffness.
Actualmente, el endurecimiento arterial se considera un buen indicador de mortalidad cardiovascular tanto en la población general como en receptores de trasplantes de renales. Para cuantificarlo, el método más empleado es la medición de la velocidad de onda del pulso carótido-femoral (VOPcf). El objetivo de este estudio es analizar si un tratamiento inmunosupresor con belatacept, sin inhibidores de la calcineurina (ICN), se asocia a una VOPcf (como marcador alternativo del endurecimiento arterial) menor que la asociada a un tratamiento con ICN. Se trata de un estudio retrospectivo caso-control, en el que incluimos todos los casos que recibieron un tratamiento inmunosupresor de mantenimiento con belatacept en nuestro centro (n=20). Para estos, seleccionamos un grupo de controles adecuado (n=20), que había recibido un tratamiento con ICN y con idénticos factores clave asociados al endurecimiento arterial. Tras el seguimiento llevado a cabo durante los 5 años posteriores al trasplante, la prevalencia de pacientes con una VOPcf superior a 8,1 m/s se había reducido en el grupo al que se le administró belatacept (50% en el grupo BLC frente al 25% en el grupo ICN, p = 0,08). El análisis de regresión logística multivariante reveló que el riesgo de presentar una VOPcf alta se encontraba correlacionado con la edad (OR: 1,24; p < 0,03) y el índice de resistencia renal (OR: 1,25; p < 0,05). El tratamiento con belatacept se asoció a una reducción significativa del riesgo de aumentar la VOPcf (OR: 0,008; p = 0,045). La inmunosupresión de mantenimiento con belatacept podría favorecer la tasa de supervivencia de receptores de trasplantes renales gracias a la disminución de los eventos cardiovasculares relacionados con el endurecimiento arterial.
INTRODUCTION
Cardiovascular disease and cancer remain the leading causes of mortality among kidney allograft recipients.1 There are some potential surrogate markers for assessing the cardiovascular risk in the clinical setting, such as arterial stiffness, which is an artery-ageing indicator as well. An increase on the stiffness causes an increase of the intra aortic pressure, which, at the microcirculation level, damages several organs like the kidneys.2 Among some surrogate measurements of the arterial stiffness, the carotid-femoral pulse wave velocity (cf-PWV) is considered an accurate index for cardiovascular risk assessment in various pathological conditions, including patients with chronic kidney disease (CKD), end-stage renal disease (ESRD)3 and kidney transplantation.4 The cf-PWV is related with age and with other cardiovascular risk factors such as arterial pressure,5 inflammation6,7 and vascular calcifications.8 However, its relationship with some classical atherosclerosis indicators, such as lipoproteins and carotid non-calcified plaques is rather low.9,10 Therefore, cf-PWV has emerged as an important predictor of cardiovascular mortality.
Regarding CKD population, there is lack of data analyzing whether kidney transplantation is capable to reduce or delay the progression of the arterial stiffness.11 The deleterious effect caused by calcineurin inhibitors (CNI) (especially cyclosporine) on endothelial cells is mainly supported by experimental studies.12 However, clinical evidence about the CNI stiffening effect in the arterial structure is quite limited13 and even controversial.14-16 Concerning mammalian target of rapamycin inhibitors (mTORi), sirolimus seems to be capable to delay the progression of arteriosclerotic injuries,17,18 whereas everolimus inhibits the fibrotic process in some tissues.19 Some authors suggest that patients treated with mTORi show a lower arterial stiffness (lower pulse wave velocity) than patients treated with CNI,20,21 although others do not agree with that conclusion.22 On the other hand, up to now any treatment is able to increase the arterial elasticity without reducing the arterial pressure4, at least in the clinical setting. Moreover, the reduction of arterial pressure caused by antihypertensive agents is associated with the reduction and/or slowing down of the progression of the arterial stiffness.23,24
The biologic immunosuppressant belatacept (BLC) is the result of a fusion between a common fragment Fc of human IgG and the CTL4 protein which, binding itself to the CD80 and CD86 receptors on antigen presenting cells (APC), inhibits co-stimulatory signals, which are essential in order to activate the T-lymphocyte. In two clinical trials recently published,25,26 belatacept has demonstrated an anti-rejection efficacy similar to cyclosporine. After a 3 years follow-up, patients treated with belatacept have shown better renal function, less renal fibrosis in protocol biopsy and a better cardiovascular profile.27 No data exist about the relationship between belatacept and arterial stiffness measured by cf-PWV.
In order to test whether a CNI-free immunosuppression based on belatacept is associated with a lower cf-PWV (as a surrogate marker of arterial stiffness) than CNI, we performed a retrospective case control study evaluating cf-PWV between a group of patients treated with belatacept (BLC) and a group treated with CNI (CNI).
MATERIALS AND METHODS
Study population
Bellvitge Hospital Institutional Review Board approved the study protocol. Pulse wave velocity measurements were performed during the first half of 2011. At this time, 24 renal allograft recipients were being treated with belatacept (BLC) as maintenance immunosuppression. As control group, we performed an individual matching (one by one) identifying in our database 26 renal allograft recipients treated with CNI during the whole the post transplant follow-up (CNI) and matched for the most important determinants of cf-PWV, which are blood pressure (the same number of medications, maximum accepted systolic and diastolic pressure difference of 5mmHg between each case and his and control, at the time of the PWV test), age (maximum accepted difference of 5 years between each case and his control), gender (matching), time since transplantation (maximum accepted difference of 6 months between each case and his control), time in dialysis (maximum accepted difference of 3 months between each case and his control), body max index (BMI – maximum accepted difference of 1kg/m2 between each case and his control) and cause of nephropathy (matching).6,13 All the patients of the two groups had given an informed consent. All the BLC patients were participants in Benefit and Benefit extended trial at our center.
Pre- and post-transplant clinical, demographic and laboratory data were obtained from patient’s charts. Inclusion criteria were renal transplantation, treatment with belatacept or CNI and informed consent. Patients were excluded from the study if there was any change in type of maintenance immunosuppression after transplantation, or they had atrial fibrillation, severe obesity (BMI >35kg/m2) and previous vascular interventions on the aorta.
Renal measurements
Graft function was assessed by an estimated glomerular filtration rate (eGFR) calculated as a MDRD formula. Proteinuria was assessed by protein/creatinine ratio and expressed as g/mol. All blood analytic tests were performed between 30 and 7 days before the hemodynamic and arterial stiffness measurements.
All patients were submitted to a standard kidney graft sonography between three and six months since transplantation. All Doppler examinations were performed by three experienced sonographers with an Anteres® machine (Siemens), equipped with 2.5-4Mhz micro-convex-array transducer (CH4-1 Transducer Siemens). For each patient, after the visualization of three segmental arteries, the Doppler spectrum was recorded and analyzed. During the examination, patients were asked to refrain from forced inspiration since this could modify the intra-abdominal pressure. The peak systolic velocity (Vmax) and end diastolic velocity (Vmin) were measured; the kidney segmental arterial resistive index (RRI) was calculated as [RI=1⁄4 1-(Vmin/Vmax)]. For statistical purposes the mean RRI of the three areas explored was used.
Hemodynamic and arterial stiffness measurements
Arterial pressure was measured, at the same time of the cf-PWV measurements, over the brachial artery three times at 5 minutes intervals with an oscillatory device (OMRON M6 Comfort HEM-7000-E). Data were presented as systolic pressure, diastolic pressure and pulse pressure (systolic – diastolic pressure). The status of arterial hypertension was defined by the use of antihypertensive medication and/or clinic blood pressure >140/90mmHg.
A unique operator (E.M.) performed the cf-PWV measurement in all patients during the morning in a quiet room, 3 hours after a light breakfast and after 30 minutes at rest. Aortic PWV was measured with the Sphigmocor® device (AtCor Medical, Sydney, Australia) by sequentially recording ECG-gated carotid and femoral artery pressure waves, using the intersecting tangent algorithm to determine the characteristic points. The path length was calculated as the 80% of the direct distance between the carotid and femoral measurement sites, as recommended by others.28 The cf-PWV was calculated as the path length divided by transit time (m/s). The cf-PWV was performed twice for each patient and the mean result was used for the analysis. The intra-observer analysis of the two measurement of pulse wave velocity was carried out with the intraclass correlation coefficient (ICC). An ICC >0.8 indicated the good liability of the test.
Statistical analysis
Results are expressed by mean ± standard deviation. Comparison between groups was performed by means of χ² de Pearson for categorical data. Fisher test was applied when number of cases was <5. The one-way analysis of variance or t-test was used for normally distributed data and the nonparametric Kruskall–Wallis or Mann–Whitney U-test for non- normally distributed variables. Univariate and multivariate cox model was used to evaluate risk factors for cf-PWV >8.1m/s. All p values were two-tailed and the statistical significance level was fixed as P <0.05. SPSS 20.0 (SPSS Inc, Chicago, IL) software was used for data management and analysis.
RESULTS
Patients
We identified a total of 24 patients treated with belatacept in our center.
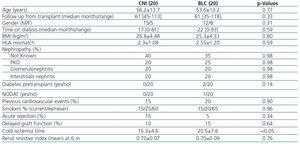
Two of them were excluded because they were on CNI treatment before conversion to belatacept. Other two, one with atrial fibrillation one with severe obesity (BMI >35kg/m2), were excluded due to technical hurdle to measure stiffness. Thus, 20 patients on BLC group were available for the study. In the CNI group 3 patients were excluded because were shift to a combination with iMtor and CNI. Other 2 patients, one with severe obesity (BMI >35kg/m2) one with a stent graft for an endovascular aneurysm repair, were excluded due to technical hurdle to measure stiffness. 1 patient in the CNI group died due to cerebrovascular accident before to perform the PWV test. So in the CNI group 20 patients remained available for the study. Patient baseline characteristics are showed in Table 1. In the CNI group, 16 were treated with tacrolimus and 4 with cyclosporine. All 40 patients were receiving mofetil mycophenolate (MMF) and 22 of them were on prednisone (5mg/day) at the time of the study. Two patients (BLC group) were diabetic before transplantation and one (BLC group) had new onset diabetes after transplantation (NODAT). Both groups were similar regarding age, gender, time in dialysis, time from transplant, acute rejection, previous cardiovascular events, BMI and cause of nephropathy. All these factors are known for their influence on arterial stiffness. However, cold ischemia time was significantly longer in the BLC group. All acute rejection episodes were mild and responded to steroid treatment.
Renal function assessment
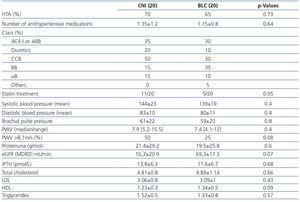
Renal function (eGFR) at the time of the study (mean 5-yr after transplantation) was numerically higher in the BLC than in the CNI group (Table 2). Also, no statistical differences were found regarding proteinuria between the two groups (Table 2). We analyzed predictors of normal renal function defined as eGFR >60mL/min by univariate logistic regression analysis. Among variables included in Table 1 and Table 2, the only predictor was belatacept-based immunosuppression (OR 0.27, IC 0.71-1.04; P=0.058 data not shown). Renal resistive index post transplantation (see material and methods) was similar in the two groups (Table 1).
Cardiovascular risk factors
Hypertension prevalence was similar in both groups. Moreover, similar findings were observed regarding the number of antihypertensive medications, mean systolic pressure, diastolic pressure and brachial pulse pressure at the time of the test (Table 2). Also, no difference in inhibitor of renin system use (both ACE-I or ARB) was seen (p. 0.73 data not shown). CNI patients needed more commonly statins to control dyslipidemia. There were no differences in total cholesterol, LDL, HDL, triglycerides and PTH between the two groups. Also, no differences in smoking prevalence were seen (Table 1).
Pulse wave velocity
The intraclass correlation coefficient for the operator (E.M.) was 0.89 (IC 0.75-0.95).
There were no differences in PWV median between the two groups 7.9±3.4m/s [Range 4.1-12m/s] in CNI group and 7.4±4 m/s [Range 5.2-15.5m/s] in BLC group (p=0.4 see Figure 1).
Due to large discrepancy of age in our population study, we chose a value of 8.1m/s of cf-PWV as cut-off value for high arterial stiffness, which was correlated with an augmented risk of cardiovascular mortality in a recent retrospective study performed in transplant population.4
The prevalence of cf-PWV above 8.1m/s was lower in the BLC group (50% in the CNI group versus 25% in the BLC group) (see Table 2 and Figure 1).
As cf-PWV higher than 8.1m/s was considered a surrogate risk factor for cardiovascular events in renal transplantation, we performed a logistic regression analysis considering cf-PWV as a categorical variable (cut-off of 8.1m/s) in order to identify predictors of high PWV in this population (Table 3). By univariate analysis, some baseline variables, such as recipient age and renal resistive index, were associated with cf-PWV >8.1m/s. Also, belatacept use tended to be associated with a low cf-PWV (OR 0.33; P=0.08). Others factors like cold ischemia time, HLA mismatch, acute rejection and BMI did not show any association with cf-PWV (data not shown). Among the variables examined when PWV test was performed, systolic blood pressure, brachial pulse pressure, statin use and proteinuria were associated with a high PWV, but not eGFR (Table 3). On the other hand, diastolic pressure, dyslipidemia (LDL, HDL and triglycerides), calcemia, phosphatemia and magnesemia were not associated with PWV (data not shown).
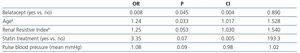
Because there is an important correlation between brachial pulse pressure and systolic blood pressure, only one of them must be selected for the multivariate model. We chose brachial pulse pressure since it has a higher correlation with cf-PWV than that observed with systolic blood pressure.2 Therefore, belatacept use, recipient age, renal resistive index, pulse blood pressure, proteinuria and statin use were considered in the multivariate logistic regression model (Table 4). Among these variables, recipient age and a high renal resistive index were independent predictors of a high cf-PWV. On the other hand, belatacept treatment was an independent predictor of a low cf-PWV.
DISCUSSION
In this study we evaluated aortic stiffness by means of a validated reference method29 in an adult renal transplant cohort with low number of previous cardiovascular events and low diabetes prevalence. Importantly, the time after kidney transplantation in the two groups was long enough to allow the evaluation of a parameter like arterial stiffness, which needs years to be modified. The major finding in our study was that belatacept treatment was associated with a lower prevalence of high arterial stiffness than CNI (see Figure 1 and Table 2). Moreover, in the multivariate analysis, belatacept treatment was an independent predictor of displaying a PWV below 8.1m/s. Although our threshold of 8.1m/s is quite lower than the 10m/s indicated by an expert consensus statement28 concerning hypertensive population, the relationship between cardiovascular risk and pulse velocity wave is continuous. Probably, in kidney transplant recipients -a group of hypertensive patients with a high cardiovascular mortality- the threshold of 10m/s is too high to best fit the cardiovascular risk, as suggested by others.4
As showed by others,2,4 also in our patients pulse wave velocity was directly correlated to arterial blood pressure (both systolic and brachial pulse pressure) and with age. Although elevated blood pressure may cause vascular damage and accelerated conduit, artery stiffening, aortic or vascular stiffness can, per se, increase pressure pulsatility and finally increase systolic blood pressure. A recent report of a Framingham study showed for the first time that a high arterial stiffness, in the long time, is a predictor of development of blood pressure hypertension and not vice-versa.30 Unfortunately we did not have basal data on blood pressure and on cf-PWV, so we could not conclude whether high stiffness was cause or consequence of the high prevalence of arterial hypertension in ours patients. In our study, hypertension prevalence was high in both groups and was quite well controlled, moreover a small difference, although not statistical significant, was seen in blood pressure measurement between the two groups. The type of CNI treatment could influence this finding, since in our study the majority of control arm patients were on tacrolimus and this CNI is more favorable than cyclosporine for controlling blood pressure.31 Thus, the fact that blood pressure was similar between belatacept and CNI groups suggests that the beneficial effect of belatacept on PWV may be independent from blood pressure control. Recently, Vinh and colleagues reported that CTLA4-Ig treatment in mice prevents angiotensin or desoxy-corticosterone acetate (DOCA)-salt-induced hypertension, and that this effect is mimicked by genetic CD80/CD86 deficiency.29 Dendritic cell can expose damage-associated molecular patterns (DAMPS) produced by vascular stress induced by mild arterial hypertension, thus promoting T cell-activation through co-stimulation signals, that can lead to a progression of stiffness and severe arterial hypertension.29 Ideally, belatacept could disrupt this cycle, blocking the costimulation of T-cell by dendritic cells.
In our study, like in the Benefit trial,26 belatacept treated patients showed a better renal function estimated by the eGFR-MDRD. In studies performed in CKD patients,2 as well as in kidney recipients,4 the GFR was inversely related to cf-PWV. In our study we have not observed any relationship between GFR and cf-PWV, probably due to the well-preserved GFR in the patients included. This result is similar to the one reported in a study including patients with diabetes and mild CKD, where no difference in PWV was seen between patients with GFR >60mL/min versus <60mL/min.32 On the other hand, the deleterious effect of arterial stiffness on renal microcirculatory system is well documented and appearance of proteinuria (or microalbuminuria) is very common in hypertensive patients.33 We found a correlation between proteinuria and PWV. However, the cause of proteinuria in renal transplant recipients is multifactorial, not just a consequence of arterial stiffness and probably this is the reason why proteinuria was not an independent predictor of PWV in the multivariate analysis.
Another interesting point of our analysis was the demonstration of a close positive correlation between resistive renal index post transplantation with a high cf-PWV. Each 0.01 reduction in the renal resistive index was associated with a reduction of 19% in the probability of having an elevated pulse wave velocity. Other authors have also demonstrated the relationship between the renal resistive index and the pulse wave velocity in hypertensive patients.33 The increase in renal resistive index represents the physiological response to a high intra-aortic pulse pressure due to high stiffness. Interestingly, renal resistive index and recipient age were independent predictors of a high pulse wave velocity, reflecting the importance of the biological (aging) status of the patient in this parameter. Among other cardiovascular risk factors, statin use was related with a high PWV in the univariate but not in the multivariate analysis, thus suggesting that patients with more vascular lesions (older and/or with high arterial stiffness) are more likely to receive a statin.
Our study has some limitations. Firstly, its cross-sectional nature, since it is not a randomized prospective trial. Secondly, we are aware of the risk of incidence-prevalence bias due to the exclusion of some patients, anyway the number of patients excluded is similar for the two study groups and only one patient in CNI group died. Thirdly, due the exclusion process, there is a little difference of prevalence (although no statistically significant) of male patients in the CNI group, possibly caused by an imbalance in favor of less stiffness in BLC group, however this difference is very small (5 vs. 8 female in CNI and BLC respectively) and probably become blurred when matching the group for other variables.
Finally, blood pressure was measured in office, and neither ambulatory blood pressure monitoring nor self-home blood pressure monitoring was performed. The fact that the difference in blood pressure was slightly inferior in the BLC group, although no significant, is another possible limitation of our study.
In conclusion, recipient age and renal resistive index were independent predictors of high arterial stiffness in kidney allograft recipients. Moreover, our study showed, for the first time, that belatacept treatment, as maintenance immunosuppression, is independently associated with low arterial stiffness long-term after transplantation. Therefore, beyond its beneficial effect on renal function, belatacept could improve kidney transplant recipient’s survival by reducing cardiovascular events related to stiffness. These results should be confirmed in a well-powered prospective trial.
Acknowledgments
This study was performed without any funding from pharmaceutical companies. This study was partly supported by the RETICS program REDinREN 12/021/0003 from the ISCIII, Spanish Government.
We thank Dr. Nuria Porta for her assistance in statistical data analysis.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Baseline patient&
Table 3. Univariate regression logistic analysis for risk of PWV >8.1ms
Table 2. Clinical, vascular and laboratory variables at the time of the study
Table 4. Multivariate regression logistic analysis for risk of PWV >8.1ms