Background: The G1 stage of chronic kidney disease (CKD) is defined in the 2012 KDIGO Guideline as kidney damage characterized by structural or functional kidney abnormalities without deterioration of glomerular filtration rate. Albuminuria and electrolyte abnormalities due to tubular disorders are considered functional markers of kidney damage. Changes in renal water handling are not explicitly cited in these guidelines. A large sample of children with abnormal dimercaptosuccinic acid (DMSA) scan located in the G1 stage was used in this study. Methods: Ambispective, cross-sectional study to evaluate the clinical histories of 116 pediatric patients. 100 patients were included in the first group (G1 stage) and 16 patients in the G2-G5 stages according to the classification of CKD Guideline KDIGO. All the patients had a renal pathologic DMSA scan. GFR, maximum urine osmolality and albumin/creatinine and NAG/creatinine ratios were determined. Results: The patients with normal GFR, in relation to those with reduced GFR, had significantly higher values of maximum urine osmolality and significantly reduced values of urine volume and albumin/creatinine and NAG/creatinine ratios. The most frequently observed alterations in children in the KDIGO G1 stage were those involving the water renal management such as urinary concentrating ability defect (29%) and increased urinary volume (20%). The frequency of children with increased urinary elimination of albumin (12%) and NAG (3%) was more lower. All children in KDIGO G2-G5 stages had alterations in water renal management. Conclusions: The parameters related with the water renal management are affected more frequently than albumin urinary excretion in children who have loss of parenchyma and normal GFR.
Antecedentes: El estadio G1 de la enfermedad renal crónica (ERC) se define en la Guía KDIGO de 2012 como el daño renal caracterizado por anomalías estructurales o funcionales del riñón y sin deterioro del filtrado glomerular. Tanto la albuminuria como las anomalías que afectan a los electrolitos debido a trastornos tubulares se consideran marcadores de daños funcionales. No obstante, en esta guía no se explicitan cuáles son los cambios que se producen en el manejo renal del agua. En este estudio, se utilizó una muestra grande de niños en estadio G1 con gammagrafías con ácido dimercaptosuccínico (DMSA) anormales. Métodos: Llevamos a cabo un estudio transversal ambispectivo para evaluar las historias clínicas de 116 pacientes pediátricos. En el primer grupo, se incluyó a 100 pacientes en estadio G1 y a 16 pacientes en los estadios G2-G5 de la ERC de la clasificación de la Guía KDIGO. Las gammagrafías con DMSA de todos los pacientes revelaban patologías renales. Se calcularon las TFG, la osmolalidad urinaria máxima y los cocientes de albúmina/creatinina y de NAG/creatinina. Resultados: En comparación con los pacientes con TFG reducidas, los pacientes con TFG normales presentaron valores de osmolalidad urinaria máxima significativamente superiores, así como volúmenes urinarios y cocientes de albúmina/creatinina y de NAG/creatinina significativamente inferiores. Las alteraciones que se observaron con mayor frecuencia en los niños en estadio G1 de la Guía KDIGO afectaban al manejo renal del agua. Entre ellas, se encontraban fallos en la capacidad de concentración de la orina (29%) y un aumento del volumen urinario (20%). Sin embargo, se observó que la frecuencia de niños en los que aumentó la eliminación a través de la orina de albúmina (12%) y NAG (3%) era mucho menor. Por su parte, todos los niños en los estadios G2-G5 de la Guía KDIGO presentaban alteraciones en el manejo renal del agua. Conclusiones: Aquellos parámetros relacionados con el manejo renal del agua se ven afectados con más frecuencia que la albuminuria en niños con pérdidas de parénquima renal y TFG normales.
INTRODUCTION
Congenital abnormalities of the kidney and urinary tract (CAKUT) are the most common cause of chronic kidney disease (CKD) in children.1,2 This etiological association is due to a prenatal reduction in nephrons and to secondary formation of renal scarring when patients suffer from one or more episodes of acute pyelonephritis.3,4 Scar nephropathy is the result of acute pyelonephritis in 25-57% of cases.5,6
In 2012 KDIGO Guideline (Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease), the G1 stage of CKD is defined by kidney damage for at least three months, characterized by structural or functional kidney abnormalities without deterioration of glomerular filtration rate (GFR).7 Markers of kidney damage are considered as one or more of the following: albuminuria, urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging and history of kidney transplantation.
Surprisingly, changes in water renal management are not explicitly cited in the 2012 KDIGO Guideline. The aim of this study is to demonstrate that the alteration of this marker is the most sensible test of kidney damage. Two parameters are used to study the renal water management and urinary excretion of albumin and NAG in a large sample of children in the G1 stage diagnosed with CAKUT and/or urinary tract infection with abnormal 99mTc-dimercaptosuccinic acid (DMSA) scan. The results were compared with those of a group of children with reduced GFR.
MATERIAL AND METHODS
Participants
An ambispective, cross-sectional study was conducted with 116 pediatric patients between 1 and 15 years of age who are followed at different stages of CKD for at least three months. The initial diagnoses were: CAKUT (n=76; 65.5%), urinary tract infection (n=31; 26.7%), anomalies of bladder function (neurogenic bladder, overactive bladder, n=6; 5.2%) and other anomalies (n=3; 2.6%). In total, 71 patients had vesicoureteral reflux (2 grade I, 7 grade II, 18 grade III, 34 grade IV, 10 grade V).
The patients were divided into two groups. There were 100 patients in G1 stage in the first group (GFR >90mL/min/1.73m2; 7.3±3.8 years; 54 male and 46 female) and 16 patients in G2-G5 stages in the second group (GFR <90mL/min/1.73m2; 7.1±4.8 years; 10 male and 6 female) according to the classification of the 2012 KDIGO guideline.7
All the patients had an abnormal DMSA scan. Table 1 contains the DMSA findings in both groups of patients.
Data collection
The inclusion criteria used to select the patients were the following: minimum one year of age, with at least one DMSA scan, one urine osmolality desmopressin test, whose glomerular filtration rate (GFR) and urinary albumin excretion had been calculated. Whenever possible, the values for N-acetyl-beta-glucosaminidase (NAG) excretion (n=67) and urine volume adjusted for 100mL GFR (V/GFR) (n=80) were recorded. These last three parameters corresponded to the first morning urine sample. Plasma creatinine values and height in cm were recorded to determine GFR by the Schwartz formula.8 V/GFR was also calculated using plasma and urine creatinine values which was obtained using the following formula: plasma creatinine x 100/urine creatinina.9,10
The biochemical parameters corresponded to the last check-up when the plasma creatinine was determined. Malformations requiring surgical attention were corrected. Patients with persistent vesicoureteral reflux and those who had suffered acute pyelonephritis within the previous two months were excluded. DMSA scans performed during episodes of acute pyelonephritis were also excluded, and only those results taken at least six months after these episodes were used.
In addition, patients were assigned to albuminuria categories as follows: A1 <3mg/mmol, A2 3-30mg/mmol, A3 >300mg/mmol.7
Desmopressin urine concentrating test
After emptying the bladder, 0.2mg (200μg) desmopressin was administered orally or 0.12mg (120μg) of desmopressin lyophilisate (MELT) was administered under the tongue.11,12 Three consecutive samples were then taken at 90 minute intervals if the patient was continent with the highest value being the maximum urine osmolality.
Outcome measures
Creatinine was determined by the creatininase method, using a Modular Analytics Analyzer (Roche/Hitachi, Mannheim, Germany). Urine osmolality was determined by freezing point depression in an Osmo Station OM-6050 osmometer (Menarini Diagnostics, Florence, Italy). Albumin was measured using a nephelometric technique (Array) and NAG activity was determined using an enzymatic colorimetric assay based on the hydrolysis of NAG-dichlorophenol sulfonephthalein (Boehringer Mannheim).
Normal values
A maximum urine osmolality value of less than 835mOsm/kg was considered to be indicative of renal concentrating defect.11,12 Urine volume was considered to be high if it was greater than 1.03mL/100mL GFR.10 Normal values for the microalbumin/creatinine ratio13,14 and NAG/creatinine ratio15,16 have been previously described.
Statistical analysis
The Kolmogorov-Smirnov test was used to examine the distribution of the sample. GFR which presented a normal distribution was expressed as mean and standard deviation. All other quantitative variables which did not present a normal distribution were expressed as median and interquartile range. Bivariate analyses were used for an initial evaluation of differences. In this sense, the Fisher’s exact test was used to compare between group frequency of qualitative variables, and the Mann-Whitney U test to compare means of quantitative variables. The Pearson test was used to calculate the correlation between quantitative variables. These analyses were carried out using SPSS statistical software (SPSS v. 19.0, SPSS Inc., USA). A P-value <.05 was considered to be statistically significant.
All of the procedures and protocols followed in this study met the ethical, administrative and data protection requirements established by the Paediatric Department of Nuestra Señora de Candelaria University Hospital, which are established in accordance with the law of Spain.
RESULTS
The biochemistry parameters corresponding to both groups are listed in Table 2. The patients with normal GFR, in relation to those with reduced GFR, had significantly higher values of maximum urine osmolality and significantly lower urine volume and albumin/creatinine and NAG/creatinine ratios.
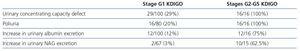
Table 3 shows that the most frequently observed alterations in children in the G1 stage KDIGO were in parameters associated with the renal handling of water, i.e., defect in urinary concentrating ability (29%) and increased urinary volume (20%). The frequency of children with higher urinary elimination of albumin (12%) and NAG (3%) was lower than the previous ones. All the children in stages G2-G5 KDIGO had alterations in the renal handling of the water. The frequency of patients with an increase in urinary albumin and NAG excretion was higher than children with normal GFR (Table 3).
As regards the category A1/G1, 23.9% (21/88) of the patients had lower maximum urine osmolality and this alteration was present in 63.6% (7/11) of the category A2/G1 and the only patient in A3/G1 (100%) (Table 4). Most of the children in stages G2-G5 KDIGO, all with defect in urinary concentrating ability, were at the A2 stage (11/16), 4 in A1 and 1 in A3.
A significant correlation was found for GFR and maximum urine osmolality (r=0.57; p<0.001).
DISCUSSION
Over the years, different screening strategies have been proposed for the detection of early CKD in the general population. Since the measurement of the glomerular filtration rate is not a very sensitive marker of initial kidney damage, the identification of sensitive markers in early stages of CKD is a priority.17,18 This search is proving to be fruitful, at least for the diagnosis of early acute kidney disease.19-21 Since the end of the 1980s albuminuria has been used as an early marker of glomerular hyperfiltration which indicated the onset of diabetic nephropathy.22-24 Subsequently, it has been observed that albuminuria rises in any cause of CKD with reduced GFR25 as well as in patients with arterial hypertension.26 However, albumin excretion is not high in all cases of CKD25 and even less when GFR is normal. Therefore, it is necessary to perform functional tests and/or the determination of other markers of renal damage, as well as the urinary elimination of albumin, to establish the involvement of CKD at an early stage.
In this study, water management by the kidneys was analyzed by determining renal urine concentrating capacity and urine volume adjusted for 100mL of GFR. Urine concentration is the result of a complex glomerulo-tubular mechanism that culminates in arginine-vasopressin (ADH)-mediated stimulation of aquaporins designed to reabsorb water in the renal collecting tubule. Renal concentrating ability depends on a proper delivery of glomerular ultrafiltrate to the tubules, a hypertonic medullary interstitium, a structurally intact countercurrent medullary mechanism and a normal water permeability of the collecting tubules in response to ADH.27 Concentrating capacity is then highly dependent on the renal medulla.28 The medullary concentration gradient is primarily established by renal tubules of the Henle loop and the blood vessels surrounding them (vasa recta) during the process of countercurrent exchange, which creates a hypertonic medullary interstitium.29 Due to that, vasopressin can concentrate the urine by passive water equilibration in the principal cells of the collecting duct, which allows the tubular lumen contents to equilibrate with the hypertonic medullary interstitium.30 Secretion of vasopressin in response to water restriction causes aquaporin2 (AQP2) to relocate from intracellular vesicles to the apical membrane of the principal cells of the collecting duct. This relocation enables water reabsorption from the urine into the cell.31 It is not surprising, therefore, that when there is a defect in any of the many factors involved in a very complex mechanism, the ability to concentrate urine deteriorates early. For this reason, in the G1-stage patients in this study, the percentage of children with the alteration of parameters that study water renal handling was higher than those who had higher albumin excretion (Table 3). The latter is only a marker for renal increasing glomerular pressure, loss of nephrons and, in some cases, of a defect in proximal renal tubular reabsorption. The ability of the kidney to concentrate urine properly is complex and, therefore, and its measurement is the first functional parameter that is altered in many kidney diseases, at least in the pediatric age.
In previous studies, we found that there is normal maximum urine osmolality in children with normal GFR, whereas all patients with impaired GFR have defects in renal concentrating capacity.10 This finding is not new. It was already pointed out by Alving and Van Slyke in 1934.32 In our experience, the renal handling of water is useful for making decisions in pediatric practice. So, we have established that cystography should not be indicated initially in cases with normal albuminuria and maximum urine osmolality in infants with mild to moderate pyelectasis (negative predictive value 93%).33 In another study, we observed that the most sensitive functional tests for detecting the loss of renal parenchyma are those which study water renal handling. However, the maximum specificity was obtained by the NAG/creatinine ratio and GFR, which were, conversely, the least sensitive tests.34
Urine volume is closely related with glomerulo-tubular function and concentrating capacity. It has been established that 99% of the fluid content in glomerular ultrafiltrate is reabsorbed along the renal tubules. When any of the different mechanisms involved in concentrating ability are altered, there is an increase in urinary volume which, however, may not be clinically detectable in mild cases. It has been known for many years that urinary volume progressively increases and concentrating ability progressively gets worse in relation to the deterioration of the GFR.35 Urinary volume corrected per 100mL GFR is seldom used in daily practice. Our results show that urinary volume corrected per 100mL GFR is an easy to calculate parameter which can be used as a marker for detecting loss of renal parenchyma, and is almost as sensitive as maximum urine osmolality.
NAG is a characteristic enzyme of renal proximal tubular cells and appears in small quantities in urine samples under normal conditions. Urinary levels of this enzyme increase when proximal tubules are damaged.16,36 The best example of increased urinary excretion of this and other low molecular-weight proteins is the administration of aminoglycoside antibiotics.37,38 We have observed, in our study, that their excretion rarely alters in children with normal GFR. Recent studies have confirmed that when GFR decreases there is progressive proximal tubular damage39 which appears to be mediated by the constitutive activation of the mTOR signaling pathway.40
We want to highlight that a possible limitation of this study is that the group of children in stages G2-G5 KDIGO is a small one.
The final conclusion of this study is that in patients with normal GFR, there can be functional renal injury, which is primarily detected by the determination of the parameters for assessing renal water management that are not defined within the definition of CKD in G1 of the 2012 KDIGO Guideline. At this stage, these parameters are more frequently affected than the urinary elimination of albumin. A normal GFR does not necessarily show normal renal function.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. DMSA scan findings in the two groups of children
Table 2. Age and biochemistry parameters in both groupsa
Table 3. Percentage of alterations in functional renal markers of kidney damage in both groups
Table 4. Concentrating capacity in the three albumin categories in children with Stage G1 of the 2012 KDIGO Guideline