Infections remain an issue of particular relevance in renal transplant patients, particularly viral infections.
Human parvovirus B19 infection causes severe refractory anaemia, pancytopenia and thrombotic microangiopathy. Its presence is recognised by analysing blood polymerase chain reaction (PCR) and by the discovery of typical giant proerythroblasts in the bone marrow.
We report the case of a 65 year-old man with a history of deceased donor renal transplant in September 2014. At 38 days after the transplant, the patient presented progressive anaemia that was resistant to erythropoiesis-stimulating agents. At 64 days after transplant, hyperthermia occurred with progressive deterioration of the patient's general condition. The viral serology and the first blood PCR for human parvovirus B19 were both negative. At 4 months and 19 days after, a bone marrow biopsy was conducted, showing giant erythroblasts with nuclear viral inclusions that were compatible with parvovirus; a PCR in the tissue confirmed the diagnosis. A second blood PCR was positive for parvovirus. After treatment with intravenous immunoglobulin and the temporary discontinuation of mycophenolate mofetil, a complete remission of the disease occurred, although the blood PCR for parvovirus B19 remained positive, so monitoring is necessary for future likely recurrence.
Las infecciones continúan siendo un problema relevante en el paciente trasplantado renal, en especial las infecciones virales.
La infección por el parvovirus humano B19 causa anemia refractaria grave, pancitopenia y microangiopatía trombótica. Dicha infección se diagnostica mediante el análisis de la reacción en cadena de la polimerasa (PCR) en sangre y por la presencia de proeritroblastos gigantes típicos en la médula ósea.
Presentamos el caso clínico de un varón de 65 años con trasplante renal de donante cadáver en septiembre de 2014. A los 38 días del trasplante comienza con anemia progresiva y resistente a los agentes estimulantes de la eritropoyesis. A los 64 días se produce hipertermia, con deterioro progresivo de su estado general. La serología vírica resultó negativa, al igual que la PCR inicial en sangre del parvovirus humano B19. A los 4 meses y 19 días se realiza una biopsia de médula ósea en la que se observan eritroblastos gigantes con inclusiones víricas nucleares compatibles con parvovirus, por lo que se realiza una PCR en dicho tejido que confirma el diagnóstico. Una segunda PCR en sangre resultó positiva. Tras el tratamiento con inmunoglobulinas intravenosas (IGIV) y la suspensión temporal del micofenolato de mofetilo, se produce una remisión completa de la enfermedad, aunque persistía positiva la PCR para el parvovirus B19 en sangre, lo que hace necesario vigilar probables recidivas.
Factors involved in the development of post-transplant anaemia are: blood loss, iron and folate deficiency, low erythropoietin, and also hyperparathyroidism, neoplasms, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers, immunosuppressive drugs, valganciclovir, co-trimoxazole, and chronic graft dysfunction1 infections caused by cytomegalovirus (CMV), Epstein–Barr virus (EBV) and parvovirus B19, which can produce bone marrow aplasia.1,2
Parvovirus B19 infection affects 40–60% of the general population, and its highest incidence is in school age children (“fifth disease”).
In kidney transplants, parvovirus B19 infection is a rare complication. Molecular biology techniques have shown viral DNA in the blood of 20–30% of transplant patients.3 Several authors have reported that the incidence of this virus in solid organ transplants is 2%.4 However, the exact incidence of infection in kidney transplants is not known, although it has been reported up to 12%.5
In healthy subjects with no transplant, parvovirus B19 is transmitted through respiratory secretions, blood and urine,6 and across the placenta to the foetus.5 In kidney transplant patients, other possibilities are secondary viral reactivation due to severe immunosuppression, transmission through blood transfusions, or even donor-derived pathways.5,7
The symptoms of parvovirus B19 infection include fever, arthralgia and rash. Other clinical changes include arthropathy, transient aplastic crisis, hydrops fetalis, abortion and foetal death; it has also been associated with vasculitis, peripheral neuropathy, myocarditis, fulminant liver failure and Nezelof syndrome.8 In kidney transplant patients, the main sign is the presence of acute or chronic aplastic anaemia.1
Here we present a short review accompanied by a case report of a kidney transplant patient who had anaemia refractory to erythropoiesis-stimulating agents given during the first few months after transplantation. Fever and general malaise subsequently appeared, and both the serological and polymerase chain reaction (PCR) tests for parvovirus B19 were negative.
Case reportWe present the case of a 65-year-old man with a history of hypertension due to primary hyperaldosteronism and chronic kidney disease since 1999, with nephrotic-range proteinuria due to chronic glomerulonephritis (no kidney biopsy). He was on haemodialysis since May 2012 until 30/09/2014, when a cadaveric kidney transplant with 6 incompatibilities was performed. The donor biopsy, with 41 glomeruli, showed that 7.3% were sclerotic glomeruli, slight hyaline thickening, less than 25% tubular atrophy and less than 5% interstitial fibrosis. Induction immunosuppressive treatment was given with basiliximab, tacrolimus, mycophenolate mofetil (MMF) and steroids.
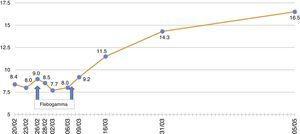
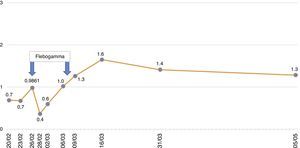
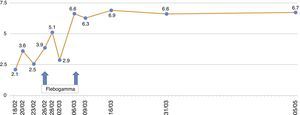
In the immediate post-transplant period, the patient had effective diuresis with no need for dialysis. In terms of complications, he developed post-transplant diabetes mellitus and haematoma from the surgical bed with development of anaemia requiring transfusion of 5 packed red blood cells. At discharge, the following values were observed: plasma creatinine (pCr): 1.56mg/dl; haemoglobin (Hb): 8.2g/dl; haematocrit: 25.5%; leukocytes: 11,300/μl; platelets: 291,000/μl. His immunosuppressive treatment consisted of prednisone 25mg/day, tacrolimus 5.5mg/day and MMF 2g/day. He was also prescribed valganciclovir and trimethoprim-sulfamethoxazole as prophylaxis. The patient progressed without symptoms, with a reduction in pCr to 1.2mg/dl in the first 15 days after the transplant. His Hb was around 8–9g/dl, with a progressive increase in erythropoietin requirements and fluctuating lymphopenia, but with no other haematological abnormalities.
Due to proteinuria of up to 1.2g/day, with no impairment in renal function, a graft biopsy was performed, this was fifty six days after the transplant (Nov/2014),. The findings were compatible with mild tubular toxicity caused by anticalcineurinics, segmental sclerosis of one glomerulus, interstitial fibrosis with mild tubular atrophy without inflammation, as well as the presence of isolated lymphocytes in the arterial intima. Immunofluorescence revealed mild-moderate IgM and C3 positivity and the C4d staining was negative. The patient was asymptomatic without impairment of renal function (pCr: 1–1.2mg/dl), with optimal immunosuppression levels, 0% anti-HLA class I and II antibodies, with no changes on the ultrasound and negative virological tests (CMV/BK). It was decided to maintain close monitoring, and a progressive reduction in urine protein was observed.
Sixty four days after the transplant (December 2014), the patient had hyperthermia, no focal deficit and a poor general condition, with asthenia, hyporexia,weight loss, with a reduction of haemoglobin to 7.1g/dl and a haematocrit of 20.6%, for which he was hospitalised. The additional tests showed no leukopenia or thrombocytopenia; normal procalcitonin levels (0.15ng/ml) and elevated LDH (321–397U/l) and C-reactive protein (7.17mg/l); total proteins: 5.7g/dl; elevated total bilirubin (1.62mg/dl) with indirect bilirubin of 0.98mg/dl; elevated ferritin (1528.60ng/ml); Na 130mEq/l, Mg 1.02mg/dl and K 3.4mEq/l, in the context of his primary hyperaldosteronism. As far as renal function, plasma creatinine did not exceed 1.05mg/dl.
During this first admission, the complete serology of the following pathogens was negative: EBV, CMV, herpesvirus, hepatotropic virus, rubella, varicella-zoster, parvovirus B19, Mycoplasma pneumoniae, Coxiella burnetii, Rickettsia typhi, Bartonella henselae, Toxoplasma gondii, leishmania, mycobacteria and fungi; as well as the blood PCR for parvovirus B19 and CMV. He had fever only the first day of admission, and thereafter the patient remained afebrile. He was treated with quinolones and third-generation cephalosporins, which were discontinued at discharge.
Normocytic, normochromic and hyporegenerative anaemia with iron overload was attributed to the blood transfusions and drug toxicity (valganciclovir and trimethoprim-sulfamethoxazole), which was decided to be discontinued.
At discharge, a serology suggested acute Q fever with positive IgM antibodies on the indirect immunofluorescence (IIF) test for Coxiella burnetii, for which treatment with doxycycline was started for 10 days. As the fever and anaemia lasted 4 months after the transplant, it was decided to admit him to hospital in order to rule out chronic Q fever.
During this second admission, chest X-ray, abdominal ultrasound, abdominal CT, PET-CT, echocardiogram, gastroscopy and colonoscopy were normal. The tumoural markers and serology tests (including new antibody assay [IIF] for parvovirus B19 and Coxiella burnetii), as well as cultures for tuberculosis and viruses (HSV-1, HSV-2, VZV, CMV, EBV, VHH-6, VHH-7, VHH-8 and enterovirus RNA), were negative in all cases. Therefore, the diagnosis of Q fever as the origin of the clinical symptoms was ruled out. It was considered a false positive, as there was no seroconversion in the second sample and the fever persisted despite treatment.
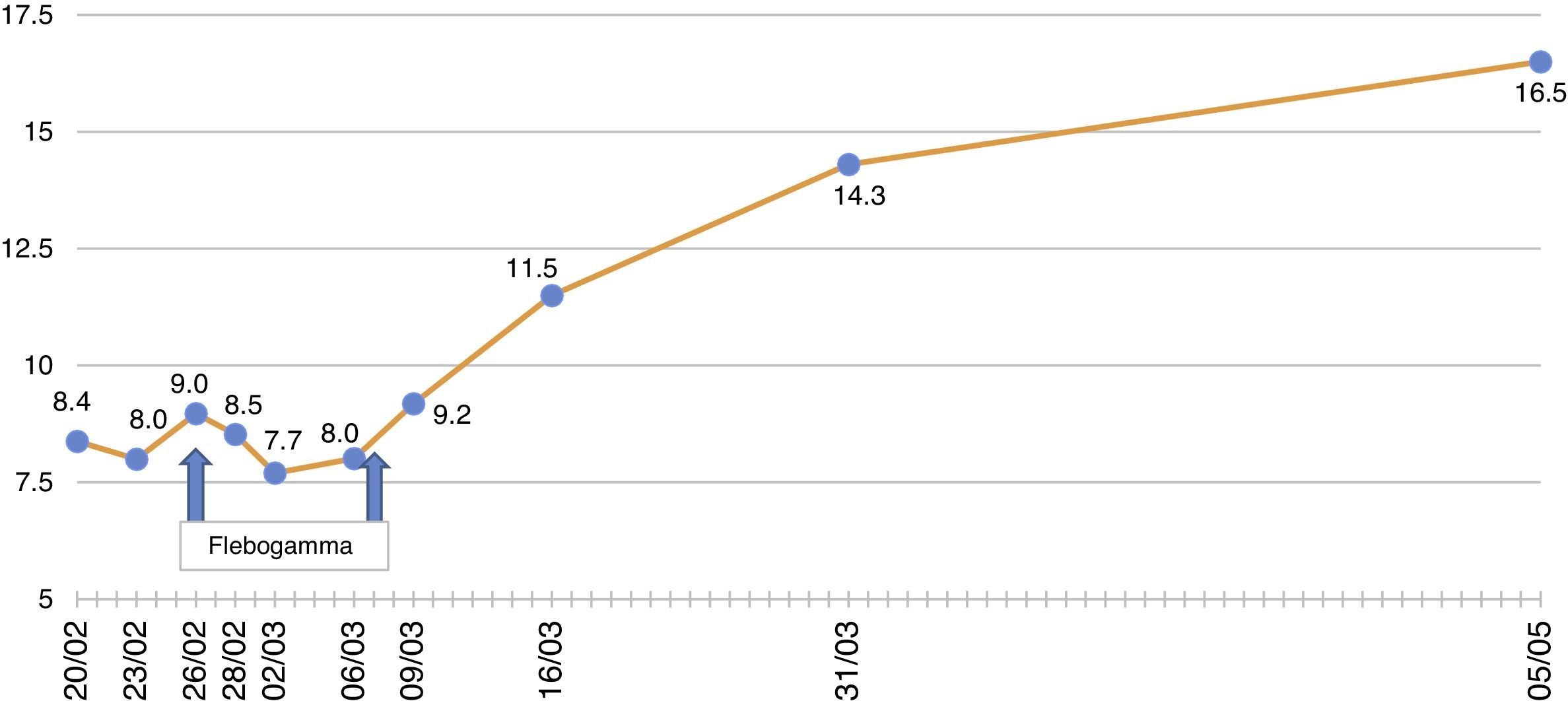
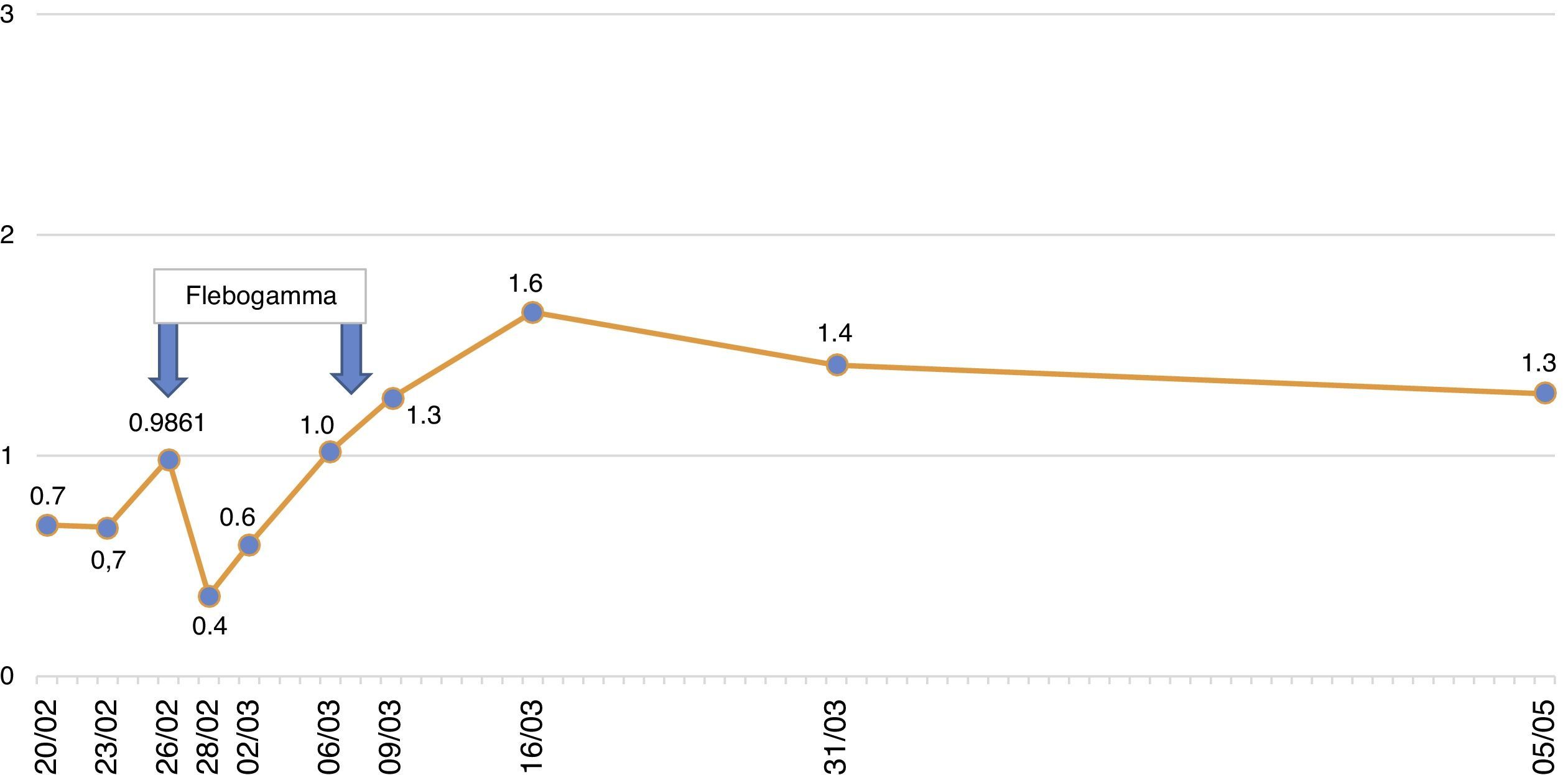
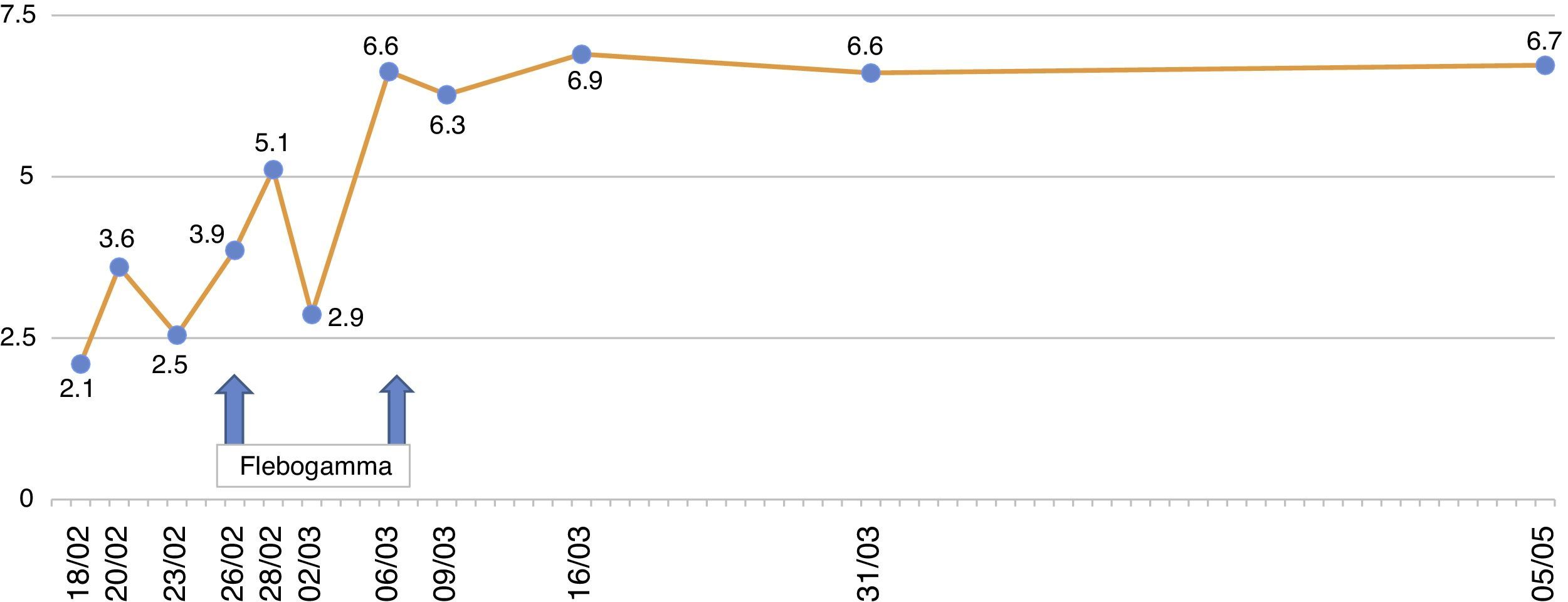
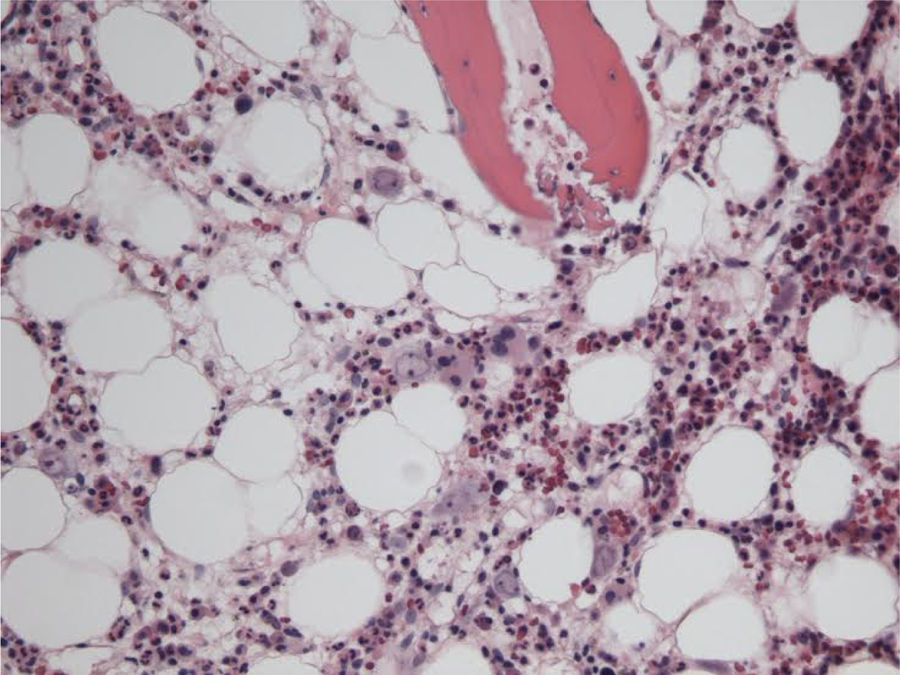
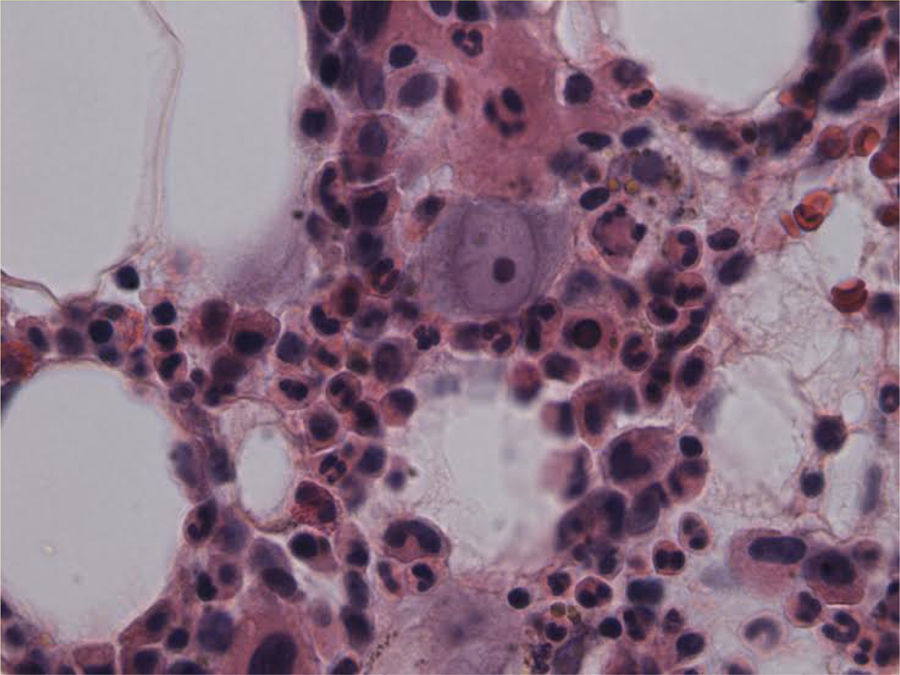
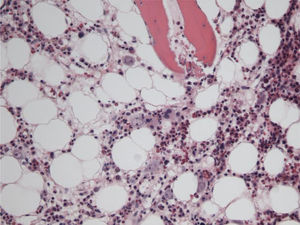
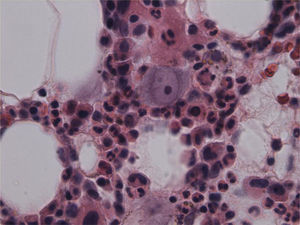
The patient remained with fever with a marked deterioration in his general condition, anaemia and lymphopenia (Figs. 1–3); it was therefore decided to perform a bone marrow biopsy on February/2015, in which hypocellular bone marrow was observed with a marked decrease of the erythroid series and with giant erythroblasts, with scarce eosinophilic nuclear inclusions that determined an increase in cell size. All of this was suggestive of parvovirus B19 infection (Figs. 4 and 5), which was confirmed by PCR with a positive result in the tissue specimen of the biopsy. Simultaneously, it was decided to perform a new blood PCR test for parvovirus B19, which this time was positive.
After the confirmation of parvovirus B19 infection, the MMF was discontinued, and treatment with a daily IV immunoglobulin 0.5g/kg/dose (10 doses) was started, with adequate tolerance, disappearance of fever, and normalisation of Hb and WBC series, after which he was discharged in March 2015.
At the end of the treatment, with stable Hb and afebrile, it was decided to resume the MMF at low doses. However, 6 months after discharge, there was a slight impairment of renal function: pCr up to 1.55mg/dl and urine protein of 2.1g/24h (previous 0.6–0.8g/24h). In considering that it could be due to the reduced immunosuppression a second kidney biopsy was performed in November 2015, which revealed mild renal tubulitis indicative of borderline acute rejection, interstitial fibrosis, moderate tubular atrophy, and areas of isometric vacuolation. There were inflammatory cells in the artery wall there were, most of which were intraparietal and a few, subendothelial. C4d staining and immunofluorescence were negative.
The parvovirus viral load was stabilised, so the immunosuppression was optimised by increasing the MMF dose from 500 to 750mg/24h and with a tacrolimus dose able to maintain levels around 7–8ng/dl, after which there was a reduction in urine protein and a stabilisation of renal function, with plasma Cr of 1.48–1.55mg/dl. The possibility of administering anti-rejection therapy was not considered since it could lead to an increase in the replication of parvovirus B19.9
A year after the end of treatment, the patient remained afebrile, with increased appetite and weight gain. With regard to the lab results, the patient had Hb levels of 15.2g/dl, with no need for erythropoietin, stable renal function, and with urine protein of less than 1g/day (controlled with dual RAAS blockade). Anti-HLA class I and II antibodies remained negative.
As for plasma PCR for parvovirus B19, our hospital's laboratory validated the quantitative method for parvovirus B19. We did not have the viral load available at the time of diagnosis, and the first quantitative measurement was done in September 2015 (approximately 7 months after the end of treatment), with <50copies/ml. We inferred that, although we were not able to completely eliminate the virus, the pre-treatment viral load was probably much larger, given the clinical and lab-value improvement observed. The current viral load remains below 100copies/ml, and clinical and lab parameters are being monitored, including periodic PCR measurements so a relapse can be detected.
DiscussionViral infections predominate in the first year after transplant, when immunosuppression is at maximum.10 Parvovirus infection in the transplanted population is rare, and the symptoms may be subtle: anaemia is the main manifestation.11
Our patient was diagnosed with parvovirus B19 infection 142 days after transplantation. Anaemia was the initial finding, which subsequently worsened; there was also hyperthermia, with a progressive deterioration in his general state, asthenia, hyporexia and weight loss.
The parvovirus infection could have been transmitted from the donor, but there was no serology or viral load available; therefore we cannot confirm transmission by this route. Another possible source of infection might have been blood transfusions during the immediate post-transplant period. It could be a reactivation of the virus in the receptor caused by immunosuppression, since we did not have the recipient's pre-transplant serology. Considering that at the time of diagnosis there was an epidemic of parvovirus B19 in the population, we suspect that our patient may have acquired the infection during this epidemiological outbreak.
The reason for anaemiaParvovirus B19 produces lysis of proerythroblast (erythroid progenitor cell).8,9 The infection of this cell occurs through the P antigen (globoside Gb4), a receptor present in erythroid cells and in others, including endothelial cells, platelets, synoviocytes, smooth muscle cells and foetal myocytes. The virus need the P antigen to bind to the cell but it is also required the presence of a co-receptor (α5β1) for the infection to be successful and to act as an integrin. Erythroid cells contain large amount of both molecules on their surface.9
Anaemia may be normocytic, severe normochromic, or with poor reticulocyte response which does not respond to blood transfusions or erythropoiesis-stimulating agents.4 Leukopenia, thrombocytopenia, and reactive haemophagocytic syndrome have also been associated to parvovirus B19 infection.12
In our patient, normocytic and normochromic anaemia was the main manifestation during the clinical course, with Hb values as low as to 6.7g/dl, with no response to erythropoiesis-stimulating agents, so he had to receive several transfusions.
DiagnosisThe diagnosis of parvovirus B19 infection is made based on the presence of persistent anaemia, usually reticulocytopenia, with giant proerythroblasts with prominent eosinophilic viral inclusions in the bone marrow, anti-parvovirus serum IgM/IgG and anti-DNA positive parvovirus PCR.13
In kidney transplant patients, the main sign is the presence of acute or chronic aplastic anaemia.1 In a study of 98 patients, the main clinical manifestations associated with parvovirus B19 infection were anaemia with associated dyspnoea and asthenia in 98% of cases, and fever in 54.9%.14 The literature on the relationship between chronic graft dysfunction and active parvovirus B19 infection is not consistent. Some authors7,15 confirm this relationship, whereas others do not.5
The serological testing needed to make the diagnosis of acute parvovirus B19 infection (IgM) has a sensitivity and specificity of 89% and 99%, respectively, in immunocompetent patients. This fact has not been confirmed in transplant patients because of their immunosuppression; in these patients the most reliable method is peripheral blood or bone marrow PCR.
The choice of diagnostic test of PCR for parvovirus B19 is important so false negatives are avoided. Some methods are sensitive for genotype 1, but not suitable for detecting genotypes 2 or 3. Therefore it is necessary to have methods able to detect the 3 genotypes.
Inhibitors in blood samples may also cause false negatives. Testing for internal inhibitors should be included when analysing the samples, to be certain that the negative result is not due to inhibitors in the sample.
In our patient, blood PCR for parvovirus B19 was first positive for 4 months and 19 days after the transplant, with a previous negative result at 73 days. The PCR method used in our hospital is the LightMix® parvovirus B19 kit, which is able to detect all 3 types of parvovirus. The fact that the first result was negative could be justified by the possibility of intermittent viraemia or a low level of viraemia at the time of the measurement.
In patients whose blood PCR is negative but in whom clinical suspicion persists, as in our case here, the definitive diagnosis of parvovirus B19 infection is performed by bone marrow aspiration.16 It shows not only a pure red cell aplasia with the characteristic medullary “shutdown” in the giant proerythroblast phase, but also, through molecular biology techniques, the virus in the marrow.3
TreatmentThere are currently no antiviral drugs that are effective against parvovirus B19. In anaemia associated with parvovirus B19 infection, different therapeutic options can be considered: decreasing immunosuppression (reduction/withdrawal of drugs), changing the immunosuppressant (substituting tacrolimus for cyclosporin – some authors have described the defective clearance of the virus in patients treated with tacrolimus17 or, in order to provide virus-neutralising antibodies,18 administering IVIG at doses of 0.4–0.5g/kg, from 2 to 10 days13 with a cumulative dose that usually ranges between 2 and 5g/kg).3 Anaemia is corrected in more than 90% of cases with only one course of treatment, but the risk of relapse ranges from 23% to 33%.19
Our patient was treated by a change in immunosuppression, temporarily suspending MMF together with intravenous immunoglobulin (IVIg; Flebogamma) daily for 10 days, with a good clinical and lab-value outcome, helping Hb, lymphocyte and leucocyte values to recover gradually, and discontinuing erythropoietin (EPO) (Figs. 1–3). Although adverse reactions to IVIg20 have been described, our patient did not have any of these disorders, and tolerated the drug very well.
Although he had a good initial therapeutic response, it is recommended to closely monitor patients where relapse and reappearance of the anaemia is possible; this would require them to be treated with a new immunoglobulin cycle every 3 months to prevent them.21
The possibility of treating these patients with mTOR inhibitors could be a promising option, but there are no current publications that support their use. In our case, we did not use it due to the post-transplant proteinuria observed, which reached 2g/24h. We did not know the specific diagnosis of kidney disease that led to haemodialysis, so the possibility of focal segmental glomerulosclerosis could not be ruled out. In addition, the histological report on the first graft biopsy reveals the presence of a glomerulus with segmental sclerosis and mild-moderate IgM and C3 positivity in 7 glomeruli. This was a reason for not using mTOR inhibitors, since focal segmental glomerulosclerosis associated with mTOR use has been described in transplant patients.
The impact of this infection on the patient's survival or long-term morbidity is not known.22 No specific strategies have proved useful in preventing parvovirus B19 infection.20
The relatively low incidence of this infection and the adverse effects of the therapeutic measures make donor/recipient screening for this virus non-viable, and the development of vaccines for parvovirus B19 is still under investigation.20
ConclusionHere it is described a kidney transplant patient with anaemia refractory to EPO and subsequent hyperthermia, with the diagnosis of parvovirus B19 infection. Due to the patient's immunosuppression, the serological anti-parvovirus IgM/IgG tests performed were always negative. Suspected acute Q fever infection delayed an accurate diagnosis, but given that there was no clinical improvement with the treatment and no seroconversion occurred, the diagnosis of Q fever infection was ruled out. Then bone marrow aspiration was performed that revealed parvovirus B19 infection, so the diagnosis was finally made since the first blood PCR did not show positive results.
Our patient was studied thoroughly, and cancer and other infections were also ruled out; the symptoms disappeared after adjusting the immunosuppressive treatment and starting IV Ig.
Conflicts of interestThe authors declare that they have no potential conflicts of interest related to the content of this manuscript.
Please cite this article as: Parodis López Y, Santana Estupiñán R, Marrero Robayna S, Gallego Samper R, Henríquez Palop F, Rivero Vera JC, et al. Anemia y fiebre en el postrasplante renal: su relación con el parvovirus humano B19. Nefrologia. 2017;37:206–212.