It is known that cerebral amyloid angiopathy (CAA) is due to amyloid beta peptide (Aβ) accumulation in the middle and outer coats of small cerebral arteries and arterioles. CAA irrupts as a intracranial hemorrhage, which can be a cerebral hematoma or multifocal microhemorrhages. It is difficult to estimate its real incidence because diagnosis confirmation requires an anatomopathological study, but it is rated that almost 57% of non-traumatic intracranial hemorrhages in patients between 71 and 80 years old might be caused by CAA.1 The spectrum of disease due to Aβ includes several disorders. In 1985, β2 microglobulin was identified as the responsible of hemodialysis-associated amyloidosis.2 In the 1980 decade, CAA was considered an Alzheimer's disease subtype,3,4 and they have been proposed as different conditions since the 1990 early decade.5 Therefore, CAA is a disease with its own entity, but it lacks diagnosis, prevention and treatment protocols.
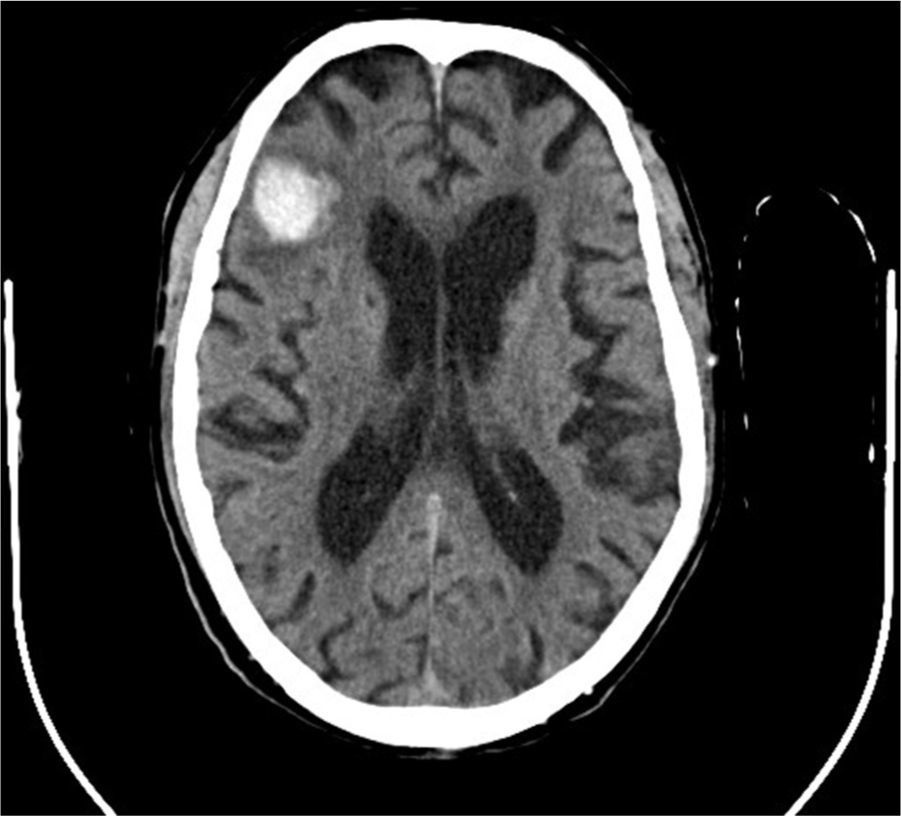
There is evidence that uremic environment and glomerular filtration rate decline in patients with chronic kidney disease, aggravate cognitive functions and are related to microhemorrhages occurrence.6 Both, it is necessary to clarify to what extent anticoagulant or antiplatelet treatments worsen CAA evolution. In this regard, it is interesting the case of an 82 years old patient with chronic kidney disease and hemodialysis for four years, who was evaluated due to a 48hours disorientation state. His antecedents included memory impairment for nine years and an episode of multiple microangiopathic infarcts eight years ago. Non-contrast head CT showed a right frontal hematoma with perilesional edema, and hypodense lesions located in periventricular and subcortical white matter and left cerebellar hemisphere. That supported former infarcts or hemorrhages. According to the Boston criteria,7 the patient diagnosis was probable CAA.
The deficiency of standardized strategies to detect CAA and the bleeding risk that the disease entails, complicate the management of concomitant pathologies with antithrombotic therapy requirement. This is particularly important in patients under hemodialysis, who need anticoagulation treatment during sessions. In regard to antiplatelet treatment, retrospective researches show different outcomes respecting bleeding risk,8 although acetylsalicylic acid is generally avoided and replaced by clopidogrel. Anticoagulation with heparin is also controversial. On the one hand, a case report of CAA-related inflammation treated with enoxaparin 4000IU/12h due to venous thrombosis, described a subsequent big cerebral hematoma which caused death.9 On the other hand, low-molecular-weight heparin (LMWH) is recommended despite cerebral hemorrhage if immobilization extends beyond 3–4 days.10 In the exposed case of the patient, LMWH treatment was kept during hemodialysis sessions, and he continued his previous therapy with clopidogrel. Six days later, a control non-contrast head TC showed the resolution of acute hemorrhage, and due to the remarkable clinical improvement, the patient was discharged from the hospital showing a normal orientation state (Figs. 1 and 2).
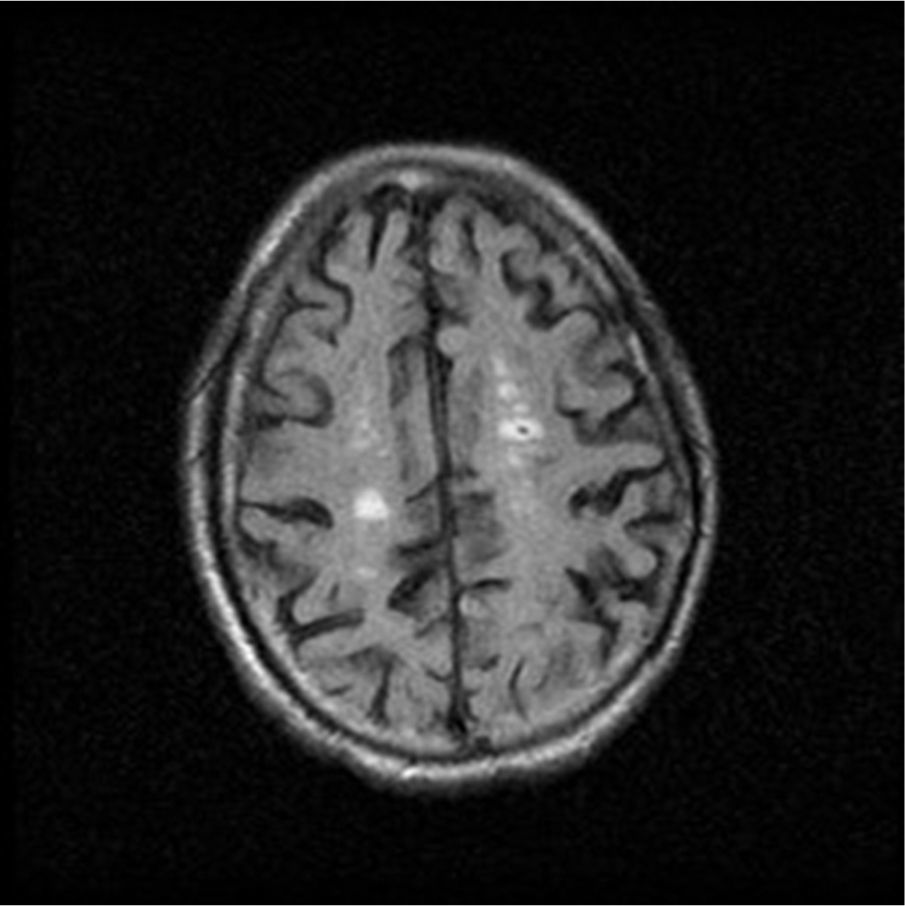
Because of its significance for hemodialysis patients, CAA is an important disease in Nephrology sphere. It is already known the importance of controlling parameters like arterial pressure, anemia, or hydroelectrolytic equilibrium in those patients in order to prevent microhemorrhages onset. However, prospective researches should focus on the bleeding risk stratification in CAA patients undergoing hemodialysis, the impact of different antithrombotic treatments in those patients, and emphasize diagnostic methods for CAA, like gradient echo sequences MRI.