Ninety percent of the cases of haemolytic uraemic syndrome (HUS) are caused by the ingestion of food contaminated with Shiga toxin-producing Escherichia coli (STEC) (typical HUS), that activates the classical complement pathway.1 There are also thrombotic microangiopathies (TMA) secondary to drugs, autoimmune diseases, and malignancies, these are related to activation of the alternative complement pathway.2–5
We present 3 patients from our hospital with advanced prostate cancer who developed severe TMA/HUS and had a good response to treatment (supportive, plasmapheresis, and haemodialysis).
A 63-year old man, with no previous history of nephropathy, who presented at the emergency room with malaise and oliguria, no GI symptoms and without fever. Past medical history and physical exam were unremarkable. The patient had acute renal failure (pCr: 6.2mg/dl), anaemia (Hb: 7.6g/dl), and thrombocytopenia (platelets: 49×109/l); elevated LDH (1281U/l), reticulocytosis, undetectable haptoglobin, and elevated number of schystocytes. Urine showed dysmorphic red blood cells, leukocyturia, and proteinuria (<1g/24h); obstructive uropathy was ruled out by ultrasound. Worsening renal function occurred at 24h, auto-immunity markers were normal, the direct Coombs test was negative, and the activity of ADAMTS13 was normal (56.9%). Faeces were not tested because of the absence of clinical signs and symptoms. The patient was diagnosed with TMA/HUS and received daily haemodialysis (HD) and plasmapheresis (PP).
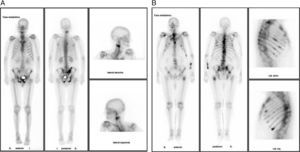
Mean while, the patient was diagnosed of prostate adenocarcinoma (PSA 195.6ng/ml and a biopsy: Gleason 9) with bone metastases (Fig. 1A). He completed 13 HD sessions, 18 PPs, and received bicalutamide, renal function and anaemia recovered, with no evidence of haemolysis (Fig. 2A). The patient was asymptomatic at the 8-month visit, and renal function, Hb, and platelets count were normal.
(A)–(C) Changes in haemoglobin, creatinine, and platelets over time in each case, concurrently with haemodialysis sessions and plasmapheresis. The first patient (1A) showed baseline pCr of 6.29mg/dl, Hb 7.6g/dl, and platelet count 49×109/l. The patient underwent HD (magenta) and subsequently received PP (blue) every day. Two weeks after beginning treatment, diuresis and renal function were gradually recovered (13 HD sessions and 18 PP sessions). Baseline pCr figures were similar for the second patient (1B), who also presented anaemia and thrombocytopenia. He received 10 and 17 HD and PP sessions, respectively, recovering one month later. The third patient (1C) had a similar behaviour, received 10 and 15 sessions, respectively, and showed recovery within one month. Hb: haemoglobin; HD: haemodialysis; pCr: plasma creatinine; PP: plasmapheresis.
An 82-year-old hypertensive COPD patient with a history of malaise, asthenia, micturition symptoms and oliguria. The patient complained of cough with production of white sputum, and a history of days of fever, with no GI symptoms or evidence volume expansion. Severe infection was ruled out and there was evidence of ARF (pCr: 7.5mg/dl), accompanied by anaemia (Hb: 12.6g/dl), and thrombocytopenia (platelets: 61×109/l), suggesting haemolysis (LDH 1600U/l, with undetectable haptoglobin and reticulocytosis). Urine specific gravity was low, and there was microhaematuria, leukocyturia, and proteinuria (<1g/24h) with no evidence of obstructive uropathy in by ultrasound. Auto-immunity markers were normal, the patient showed increased schystocytes, negative direct Coombs test, and preserved ADAMTS13 activity (25.4%). Faeces were negative for STEC. After confirming TMA/HUS, the patient received HD and PP (Fig. 2B) and was diagnosed with advanced prostate cancer (PSA: 760ng/ml with bone metastases, Fig. 1B), initiating treatment with bicalutamide. A month later his renal function had normalised, with discrete thrombocytopenia, and anaemia (Hb: 10.5g/dl) (Fig. 2B).
An 89-year old men with diabetes and benign prostate hyperplasia. He seek medical help because general deterioration, abdominal pain, macroscopic haematuria, and oliguria during the last a 6-days. No respiratory, cardiac, or gastrointestinal symptoms a physical exam without findings. The patient developed acute renal failure (pCr: 9.4mg/dl), with haematuria and non-nephrotic proteinuria; haemolytic anaemia (Hb: 10.1g/dl, reticulocytosis, LDH: 4411U/l, haptoglobin: 54mg/dl, increased schystocytes) and severe thrombocytopenia (platelets: 13×109/l). Obstructive aetiology of acute renal failure was ruled out by ultrasound. ADAMTS13 activity was preserved. The patient was diagnosed of TMA/HUS, and was treated with HD and PP for 3 weeks, with subsequent improvement of renal function, thrombocytopenia, and anaemia (Fig. 2C). The patient was diagnosed of prostate cancer and received specific treatment; the renal function did not recover totally and remained with some residual renal failure.
In our three patients with TMA/HUS, disseminated intravascular coagulation (DIC) was ruled out and the diagnosis was made on the basis of the clinical history and precipitating causes, since signs and symptoms of aHUS are unexpectedly non-specific according to the consensus guidelines published in Nephrology (2013).6
Most cases of TMA/HUS are caused by STEC infections. Yet, there have been reports of TMA/HUS secondary to drugs, autoimmune diseases, or malignancies.5,7,8 In patients from oncologyin, the most common cause of TMA/HUS is in association with chemotherapy drugs (mainly mitomycin), whereas cases directly associated with malignancies are less common. The largest series available consists of 168 patients; being GI tumours are the most common kind (26%), followed by breast and prostate cancer,9 thereby stressing the distinctiveness of our report.
Complete response to classical treatment for TMA/HUS (PP, HD, and supportive) was observed in the cases presented here. Based on good response to treatment with PP, the deregulation of the alternative complement pathway, either resulting from individual susceptibilities because of mutations in regulatory proteins, or the presence of antibodies against them, may lead to this condition.4 Resistance has also been reported with a positive transient response to anti-C5a (eculizumab).10
The association of TMA/HUS to malignancies complicates the patient's outcome; the therapy employed against neoplasias are no always responsible for TMA/HUS. Early diagnosis and treatment of TMA/HUS may lead to resolution with no sequelae. The thriving research concerning the complement will help identify the causes and the right treatment for each patient.
Please cite this article as: Rullan M, Manrique J, Lorente LF, Izquierdo D, Slon F, Rullan AJ. Síndrome hemolítico urémico como forma de presentación de cáncer de próstata diseminado. Nefrologia. 2016;36:194–196.