We present a case of acute interstitial nephropathy (AIN) associated with Fanconi syndrome, resolving after early treatment with steroids.
A 49-year-old man with a past medical history of alcoholic pancreatitis and liver disease, hypertriglyceridemia, and hypertension (HTN) treated with gemfibrozil and valsartan for over one year.
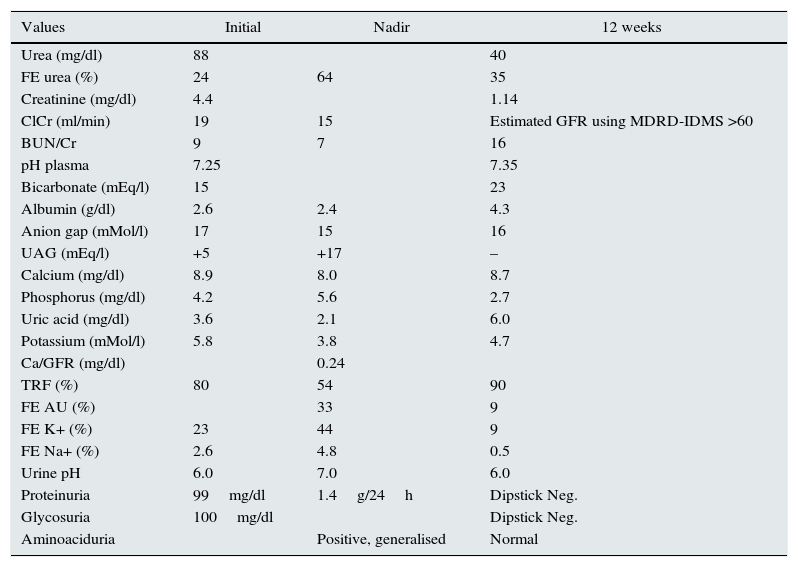
Two months ago, he had undergone an umbilical hernia repair. At that time, plasma creatinine was 0.79mg/dl and urinalysis was normal. He received a cephalosporin, and 3 weeks of analgesia in the form of metamizole and paracetamol, with complete recovery. Two weeks later, metamizole was restarted at a dose of 575mg/8h orally for flu-like symptoms. He developed progressive weakness, abdominal pain, and nausea. Oral intake was reduced and BP control worsened, therefore the doses of valsartan and metamizole were increased, with additional paracetamol as required. His general condition worsened, therefore he attended the emergency department where BP was 135/85. Skin and mucous membranes were dry, he was afebrile, with no skin lesions, and no other physical findings. He had a high urine output (3.5L/day), which was macroscopically normal. Blood tests showed a full blood count with no eosinophilia, metabolic acidosis with normal lactic acid, normal CK, reduced glomerular filtration rate (GFR), hypouricemia, and a mixed proteinuria of 1.4g/24h. Urinalysis revealed an alkaline pH, glycosuria with normoglycaemia, microhaematuria, isosthenuria, and the presence of granular hyaline casts.4,5 There was reduced tubular reabsorption of phosphate, potassium, calcium, and uric acid, and generalised hyperaminoaciduria. Abdominal ultrasound showed kidneys measuring 146mm with preserved corticomedullary differentiation and no signs of hydronephrosis. There were no abnormalities of the bladder or prostate. Viral serology and tumour markers were negative. Immunology was normal, including complement, ANA, ANCA, anti-GBM, and protein electrophoresis and immunoglobulins. Intact parathyroid hormone, 72pg/mL; 25-OH-vitamin D, 6.0ng/mL; ACE, normal Mantoux negative; and CXR, normal (investigation results, Table 1). Pre-renal factors were corrected, but with no improvement in GFR, therefore renal biopsy was performed. This showed 13 glomeruli with no abnormalities, interstitial oedema, an intense lymphoid infiltrate accompanied with polymorphonuclear neutrophils and eosinophils, no granuloma, acute tubular necrosis, normal arteries and arterioles, and no complement or immune-complex deposits on direct immunofluorescence. A diagnosis was made of acute renal failure due to tubular necrosis and acute interstitial nephritis (AIN) secondary to metamizole, and Fanconi syndrome. Treatment was started with methylprednisolone 250mg/day for 3 days and was then switched to prednisone 1mg/kg/day at a reducing dose until it was stopped 12 weeks later, once the GFR had recovered and the tubular abnormalities had resolved.
Investigation results.
| Values | Initial | Nadir | 12 weeks |
|---|---|---|---|
| Urea (mg/dl) | 88 | 40 | |
| FE urea (%) | 24 | 64 | 35 |
| Creatinine (mg/dl) | 4.4 | 1.14 | |
| ClCr (ml/min) | 19 | 15 | Estimated GFR using MDRD-IDMS >60 |
| BUN/Cr | 9 | 7 | 16 |
| pH plasma | 7.25 | 7.35 | |
| Bicarbonate (mEq/l) | 15 | 23 | |
| Albumin (g/dl) | 2.6 | 2.4 | 4.3 |
| Anion gap (mMol/l) | 17 | 15 | 16 |
| UAG (mEq/l) | +5 | +17 | – |
| Calcium (mg/dl) | 8.9 | 8.0 | 8.7 |
| Phosphorus (mg/dl) | 4.2 | 5.6 | 2.7 |
| Uric acid (mg/dl) | 3.6 | 2.1 | 6.0 |
| Potassium (mMol/l) | 5.8 | 3.8 | 4.7 |
| Ca/GFR (mg/dl) | 0.24 | ||
| TRF (%) | 80 | 54 | 90 |
| FE AU (%) | 33 | 9 | |
| FE K+ (%) | 23 | 44 | 9 |
| FE Na+ (%) | 2.6 | 4.8 | 0.5 |
| Urine pH | 6.0 | 7.0 | 6.0 |
| Proteinuria | 99mg/dl | 1.4g/24h | Dipstick Neg. |
| Glycosuria | 100mg/dl | Dipstick Neg. | |
| Aminoaciduria | Positive, generalised | Normal |
Acute interstitial nephritis comprises 15–27% of findings in renal biopsy.1 Recent years have seen a change in the clinical pattern and causative agents.2 The observed incidence of AIN secondary to metamizole is 10.48 cases per 10,000 patient/year.1 Although previously described,3 it is not common to find a Fanconi-type proximal tubulopathy.
In this case, features of AIN were found (isothenuria, non-nephrotic proteinuria, rapid deterioration of GFR) along with features typical of a proximal tubulopathy (metabolic acidosis, glycosuria with normoglycaemia, hyperphosphaturia, hyperuricosuria, and generalised hyperaminoaciduria). The main mechanism was direct immune-mediated tubular toxicity related to metamizole. The second mechanism was tubular necrosis secondary to renal vasoconstriction caused by the inhibition of prostaglandins by metamizole (inhibition of the enzyme COX-3) and gemfibrozole (stimulation of the peroxisome prolipherator-activated receptor PPAR-α6,7), and by the inhibition of angiotensin II by valsartan. As in other studies,4 renal failure developed between 1 and 3 weeks after taking the drug, was nonoliguric, without eosinophilia, skin changes, or fever, and with characteristic abdominal pain and proteinuria of around 1g/day. The absence of deposits on DIF and the fact that the patient developed symptoms on the second exposure to the drug led us to think of an IgE-mediated type I hypersensitivity mechanism.
Notably, there was an increased anion gap (AG), which was unexpected for a proximal renal tubular acidosis. We ruled out consumption of salicylates, ethanol, propylene glycol and ethylene glycol, methanol, toluene, and other causes that would explain l- and d-lactic acidosis. There was no evidence of rhabdomyolysis, and no measurable changes in plasma calcium, magnesium, or albumin levels. We did not find any evidence in the medical literature that metamizole, valsartan, or gemfibrozole increased AG by producing intermediate metabolites. It is possible that the consumption of paracetamol, by producing the metabolite 5-oxoproline, would explain the finding.8,9 In fact, the patients had consumed it prior admission, during his hospital admission and after discharge. The acquired accumulation of 5-oxyproline is found in long-term paracetamol users, and occurs with normal blood paracetamol levels. Predisposing circumstances are female sex, malnutrition, pregnancy, vegetarian diet, sepsis, chronic renal failure, liver disease (mainly alcoholic), and the use of certain antibiotics and anticonvulsants.
The combined action of drugs that synergistically affect the distal tubule (valsartan) and proximal tubule (metamizole and genfibrozil3,7) could increase the chances of renal damage. This should be taken into account at the time of prescribing them concurrently. It is worth considering the possibility that the fibrate might have played a role in the development of Fanconi syndrome, given its action on the proximal tubule, although the timing of the clinical presentation makes this less likely.
FundingThe authors declare that they have received no funding for this work.
Please cite this article as: Martín-Navarro JA, Petkov-Stoyanov V, Gutiérrez-Sánchez MJ, Pedraza-Cezón L. Fracaso renal agudo por nefritis intersticial aguda con síndrome de Fanconi en relación con metamizol y gemfibrozilo. Nefrologia. 2016;36:321–323.