Introduction: During the last years the number of patients on waiting list for kidney transplantation has been stable. Living donor kidney transplantation is nowadays a chance to increase the pool of donors. However, there are a group of patients with ABO incompatibility, making impossible the transplant until now. The aim of the present study is to describe the experience of Hospital Clinic Barcelona on ABO incompatible living transplantation. Material and methods: A retrospective-descriptive study was made based on 11 living donor kidney recipients with ABO incompatibility in Hospital Clinic of Barcelona from October’06 to January’09. Selective blood group, antibody removal with specific immunoadsortion, immunoglobulin and anti-CD20 antibody were made until the immunoglobulin (IgG) and isoaglutinine (IgM) antibody titters were 1/8 or lower. Immunosuppressive protocol was adjusted to particular recipient characteristics. Isoaglutinine titters were set before, during and post desensitization treatment and two weeks after transplant. Immunological, medical and surgical evaluation was the standard in living donor kidney transplant program. Results: Medium age of donors and recipients were 47.8 ±12.4 and 44.4 ± 14.1 years, respectively. 90% of donors were females and 73% of recipients males. Follow-up time was 10.2 ±10.2 months. Siblings and spouses were the most frequent relation (n = 4, 36.4%, respectively). Chronic glomerulonephritis, adult polycystic kidney disease and Alport syndrome, the most frequent cause of end-stage renal disease. All the patients acquire appropriate isoaglutinine titters pre transplant (<1/8), requiring 5.54 ± 2.6 immunoadsorption sessions pretransplant and 2.82 postransplant. One patient didn´t need any immunoadsorption session (incompatibility blood group B) and another patient plasma exchange instead of immunoadsorption for being hipersensitized with positive flow cytometry crossmath. Postransplant isoaglutinine titters remained low. Two patients had cellular acute rejection episode (type IA and IB of Banff classification) with good response to corticosteroid treatment. Patient and graft survival were 91% at first year and remain stable during the follow-up. A graft lost by death of patient in relation to haemorrhagic shock developed within the first 72 hours after transplantation. Renal graft function at first year was excellent with serum creatinina of 1.3 ± 0.8 mg/dl, creatinine clearance of 62.6 ml/min/1.73 m2 and proteinuria of 244.9 mg/U 24 h. Conclusion: ABO incompatible living donor kidney transplantation represent an effective and safe alternative in certain patients on waiting list for renal transplant, obtaining excellent results in patient and graft survival, with good renal graft function.
Introducción: En los últimos años se ha mantenido estable el número de pacientes en lista de espera para un trasplante renal. El trasplante renal de donante vivo representa actualmente una vía para aumentar el pool de donantes, pero hay un grupo de pacientes que presentan incompatibilidad de grupo sanguíneo ABO, lo que contraindicaba hasta ahora que pudiera llevarse a cabo el trasplante. Nuestro objetivo consiste en describir nuestra experiencia con el programa de trasplante renal de donante vivo con incompatibilidad de grupo ABO. Material y métodos: Se trata de un estudio de retrospectivo-descriptivo de los primeros 11 pacientes sometidos a trasplante renal de donante vivo ABO incompatible en el Hospital Clínic de Barcelona desde octubre de 2006 a enero de 2009. Se utilizó un protocolo de acondicionamiento basado en inmunoadsorción específica (con número sesiones necesarias hasta conseguir títulos de isoaglutininas aceptables pretrasplante), inmunoglobulina policlonal inespecífica y anticuerpo monoclonal anti-CD20, seguido del tratamiento inmunosupresor adaptado a cada receptor. Se determinaron títulos de isoaglutininas antes del tratamiento de acondicionamiento, pretrasplante y postrasplante durante las primeras 2 semanas. La valoración inmunológica, médica y quirúrgica fue la habitual en el programa de trasplante renal de donante vivo. Resultados: La edad media de los donantes y receptores fue de 47,8 ± 12,4 y 44,4 ± 14,1 años, respectivamente. Un 90,1% de los donantes fue mujer y un 72,7% de los receptores, hombres. El tiempo de seguimiento medio fue de 10,2 ± 10,2 meses. Hermanos y esposos fueron las relaciones más frecuentes (n = 4, 36,4%, respectivamente), al igual que la causa de nefropatía fueron la glomerulopatía, poliquistosis y el síndrome de Alport (n = 2, 18,2% para cada enfermedad renal primaria). Todos los pacientes adquirieron un título de isoaglutininas correctos pretrasplante (<8) y requirieron 5,54 ± 2,6 sesiones de inmunoadsorción pretrasplante y 2,82 sesiones postrasplante. Un paciente no requirió ninguna sesión de inmunoadsorción (única con incompatibilidad anti-B) y otro requirió recambios plasmáticos, en vez de inmunoadsorciones, por tratarse de un potencial receptor hipersensibilizado con crossmatch por citometría de flujo positivo. Los títulos de isoaglutininas postrasplante se mantuvieron a títulos bajos. Dos pacientes presentaron un episodio de rechazo agudo celular (Banff IA e IB), con buena respuesta al tratamiento. La supervivencia de paciente y del injerto fue de un 90,9% en el primer año y se mantuvo estable a lo largo del seguimiento. Únicamente se registró una pérdida del injerto por fallecimiento en relación con una complicación hemorrágica en las primeras 72 horas sin relación con la incompatibilidad de grupo ABO. La función de injerto renal al año es excelente, con valores de creatinina sérica de 1,3 ± 0,8 mg/dl, con aclaramiento de creatinina ajustado a superficie corporal 62,6 ml/min/1,73 m2 y proteinuria de 244,9 mg/orina de 24 horas. Conclusiones: El trasplante renal de donante vivo con incompatibilidad de grupo sanguíneo representa una alternativa eficaz y segura en determinados pacientes en lista de espera de trasplante renal, obteniendo resultados excelentes de supervivencia de paciente e injerto y con una buena función de injerto renal.
INTRODUCTION
Kidney transplantation is considered to be the best treatment alternative for patients with chronic end-stage kidney failure since it offers the best survival rates and the greatest improvement to quality of life.1,2 In recent years, we have seen the number of patients on the transplant waiting list come to a standstill, despite our efforts to develop procedures that would allow more patients to receive transplants. However, the current problem with waiting lists is that for many patients, due to their surgical difficulty or age-related, immunological, or cardiovascular characteristics, a live-donor kidney transplant may be the only transplant option.3
With the increase in live-donor kidney transplant programmes, it is common for a donor to be ABO incompatible despite having a high degree of HLA compatibility. Therefore, when this situation exists, a good alternative is performing a live-donor kidney transplant with ABO incompatibility.4-6
In 1974, Gelin and Sandberg7 were the first to show that an ABO kidney transplant was possible. They performed 21 cadaver donor transplants of A2 blood type kidneys for O type recipients. No hyperacute rejection occurred and the mean survival for the kidney grafts was similar to that for ABO-compatible kidney transplants performed at that time.8,9 Since then, numerous groups have worked hard to develop treatments that permit elimination of anti-A/anti-B antibodies so that it would be possible to perform immunologically safe ABO-incompatible kidney transplants.10-13
Current protocols for ABO-incompatible renal transplants are based on a combination of different therapeutic procedures. Different protocols are found in the literature, and they have changed throughout the history of this type of transplant. Traditionally, plasma exchange11,12 has been used to reduce the level of circulating isoagglutinin. More recently, Europe has seen the introduction of an antigen-specific immunoadsorption technique for anti-A/anti-B antibodies (ASI) using adsorption columns (Glycosorb).14-18 One thing that is common to nearly all groups is administration of polyclonal immunoglobulins (IVIG); the difference is that splenectomy has now been abandoned19,20 in favour of using anti-CD20 monoclonal antibodies (Rituximab, RTX).15,16 However, some groups, Tanabe’s group in Japan19,20 do continue practicing splenectomy (although it is less common) since RTX is not covered by the Japanese national health care system. The immunosuppressant treatment used is no different from that employed in any ABO-compatible kidney transplant.16,20
The objective of this study is to present the first results obtained from our ABO-incompatible kidney transplant programme, and to reflect on how the action protocol has changed with the accumulation of experience.
MATERIAL AND METHOD
Study population
Between October 2006 and January 2009, 11 out of the total of 84 live-donor kidney transplants performed in Barcelona Clinical Hospital were ABO blood-type incompatible. All of the patients followed the pre-conditioning and desensitisation protocol approved by the Medical Ethics Committee at Barcelona Clinical Hospital, and they gave their informed consent.
Pre-conditioning protocol
The protocol used for this type of transplant is shown in figure 1. Here we see the protocol that was initially accepted in our centre, and which is based on a pre-transplant conditioning treatment with a single dose of RTX (anti-CD20 monoclonal antibody, MabThera, Roche®, Nutley, NJ, USA) dosed at 375mg/m2
and administered on the eighth day before the transplant; four sessions of ASI (Glycosorb ABO, Glycorex®, Lund, Sweden); and a dose of IVIG (Flebogamma IV 5%, Grífols Institute®, Barcelona, Spain) before the last ASI session. This was followed by three ASI sessions during the first week after the transplant. Depending on the isoagglutinin titre levels, more ASI sessions were added until proper isoagglutinin titres were obtained. After gaining experience with the first transplants, the initial protocol was modified (as shown in figure 1 with dotted arrows) by administering anti-CD20 monoclonal antibody at an earlier point, increasing the number of ASI sessions to five, and dividing the same IVIG dose between two sessions administered four days and one day before the transplant. In cases where there was immunological sensitisation and a current positive-flow cytometry crossmatch, plasma exchanges were used instead of ASI. Splenectomy was not needed in any of the cases.
For anti-A or anti-B ASI, we used blood type A or B antigen columns with the trisacharide responsable for A or B blood type specificity attached covalently to sepharose particles.14 Before being placed in the column, the plasma was separated using a cellular separator (Cobe Spectra, Lakewood, Co., USA). The anticoagulant used was sodium citrate (ACD-A) reverted with a solution of calcium chloride and magnesium sulphate in a proportion of 1M divalent ions per 10M of citrate. In each ASI session, between 2 and 3 volumes of plasma were passed through the column. Three columns were used per patient; these were conveniently regenerated and preserved after each usage.21
Titre monitoring
Isoagglutinin titration was carried out according to the Hemotherapy and Hemostasis Unit standard techniques. Whenever it was possible, the titration employed red blood cells from the kidney donor in a 3% dilution in physiological saline solution. The IgM titre was estimated using a saline method in a gel card (Reverse Diluent cards, Ortho-Clinical Diagnostics, Raritan, NJ, USA). 10µl of the red blood cell solution was added to 40µl of the corresponding diluent. After a ten-minute incubation period, the card was centrifuged for ten minutes and then read. The IgG titre was estimated using an antiglobulin technique with a gel card (Anti IgG-C3d, Polyspecific, Ortho). 40µl BLISS, 10µl red blood cell solution and 40µl of the corresponding diluent was dispensed into each microtube on the card. It was incubated at 37º C for 15 minutes, centrifuged for five minutes, and then read. The titre was considered as the inverse of the last solution for which an agglutination intensity of +2 or more was observed. The isoagglutinin titres were systematically evaluated pre- and post-ASI, and underwent additional evaluations if it was clinically required. Patients with IgG or IgM isoagglutinin titres above 512 before the transplant were excluded from the transplant programme.
Immunological parameters and immunosuppresant protocol employed
When evaluating immunological parameters, we followed the centre’s protocol for any live-donor kidney transplant.22,23 A cytotoxicity method was used to measure the anti-HLA titre (panel-reactive antibodies, PRA). A cross-test was performed using lymphocytotoxicity and flow cytometry techniques; the first technique required a negative test, and in the event of a positive flow cytometry crossmatch, a total of six plasma exchange sessions were performed instead of ASI, with three IVIG doses and two RTX doses before the transplant. The immunosuppressant protocol used for this type of transplant was based on quadruple immunosuppressant treatment with tacrolimus, mycophenolate mofetil/mycophenolate sodium and prednisone with use of induction therapy with monoclonal (basiliximab) or polyclonal (rabbit antithymocyte globulin) antibodies depending on prior immunological sensitisation, crossmatch, and the potential recipient’s historical and current PRA titres.22
Parameters for kidney graft function, histopathology and antimicrobial prophylaxis
Kidney graft function was evaluated according to plasma creatinine values (in mg/dl), urine creatinine clearance over 24 hours adjusted by body surface area, glomerular filtration rate calculated by MDRD (measured in ml/min/1.73m2) and proteinuria in urine collected in the 24 hours before analysis (defined as clinical where values exceed 300mg/24-hour urine volume). Delayed kidney graft function was defined as a drop in plasma creatinine of less than 20% in the first 48 hours following the transplant. The Banff24 classification was followed when evaluating histopathological data obtained from renal graft biopsies. Routine biopsies were not performed, as this strategy has not been used in our centre up to now.
Rutine cytomegalovirus prophylaxis was adopted for high risk patients according to donor’s and recipient’s serological status, and anti-Pneumocystis jiroveci prophylaxis was administered to all patients.
Statistical study
Statistical analysis was carried out using non-parametric descriptive and frequency tests. The results are shown as means ± standard deviations. P-values less than 0.05 are considered significant. To carry out the analysis, we used SPSS statistical software (version 14.0, SPSS Inc.®, Chicago, IL, USA).
RESULTS
Demographic characteristics
The mean recipient age at time of transplant was 44.4 ± 14.1 (26-70) years, while donors had a mean age of 47.8 ± 12.4 (27-63) years at the time of donation. Most of the donors were women (90.1%), while more men were recipients (72.7%). Siblings and spouses accounted for the most common donor/recipient relationships at 36.4%, followed by mother/child at 18.2%. In 18.2% of the recipients, the most prevalent primary renal diseases were glomerular disease, renal polycystic disease and Alport syndrome; 36.4% were in predialysis, 54.5% on haemodialysis and only one patient was on peritoneal dialysis.
The mean HLA compatibility was 2.09 ± 1.81; one recipient-donor pair had identical HLA and two did not share any HLA antigens. 18.2% were considered hypersensitive (PRA > 50%) and 72.7 were not sensitized (PRA < 10%). One pair had a historical and current positive flow-cytometry crossmatch, which gave a negative result using lymphocytotoxicity. 27.3% of the patients received antithymocyte globulin induction, and the rest received basiliximab. Donors and recipients characteristics are shown in Table 1.
ABO conditioning protocol
All recipients who initiated preconditioning treatment reached pre-transplant isoagglutinin titres considered to be acceptable in order for the transplant to be carried out (IgM and IgG < 8). Two pairs were previously eliminated due to high isoagglutinin titres before the preconditioning phase. On average, 5.5 ± 2.6 ASI sessions were carried out in order to reach this pre-transplant titre range; recipients in pairs 1, 2, 6 and 11 were the ones who had to have the most sessions, since their baseline isoagglutinin titres were higher. The recipient in pair 10, the only one to have anti-B incompatibility, did not require any ASI sessions due to very low initial isoagglutinin titres (IgM and IgG at 4), but did receive the corresponding IVIG and RTX doses. The recipient in pair 7 received the pre-transplant desensitisation protocol with plasma exchange instead of ASI in addition to three IVIG and two RTX doses. This is because the patient was hypersensitive with a historical and current positive flow-cytometry crossmatch. The crossmatch became negative and the isoagglutinin titres were significantly reduced, as occurred with patients undergoing ASI sessions.
An average of 2.8 ASI sessions were needed post-transplant, with the particular exceptions of the recipient in pair 9, who died 72 hours after the transplant and only received one session; the recipient in pair 8, who underwent no post-transplant sessions due to negative isoagglutinin titres pre-transplant and this situation continuing after the transplant; and the recipient in pair 10 who did not undergo any pre-transplant sessions, for the same reason explained in the above section. The isoagglutinin titres were monitored during the post-transplant period and remained low during the first 15 days after the transplant (table 2).
Patient and graft survival and acute rejection episodes
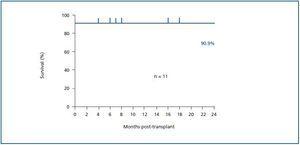
One patient died of hypovolemic shock in the third day after the transplant, which was probably due to suture dehiscence in the vascular anastomosis. The patient had an aorto-bifemoral bypass with a Dacron prosthesis. The other ten recipients are alive and with functioning grafts at present; follow-up time was 10.2 ± 10.2 months (figure 2). Two patients (18.2%) presented acute rejection during the early post-transplant months; both were cell type IA and IB according to the Banff classification,24 and both responded to treatment with methylprednisolone boluses. There were no episodes of acute late-onset rejection or acute humoral rejection.
Kidney graft function
All of the patients demonstrated immediate improvement in kidney graft function from the initial moment (including the patient who died 60 hours afterwards); there were no delayed graft function episodes. Plasma creatinine after 48 hours was already 2.49 ± 1.31mg/dl on average (starting from values of 6.29mg/dl prior to the transplant), and values at time of discharge were 1.2 ± 0.28mg/dl. These have remained stable between 1.3 and 1.4mg/dl during the follow-up period (figure 3), and so has proteinuria (244.9 ± 213.4 and 218 ± 106.1mg in 24-hour urine volume at 12 and 24 months, respectively). Only one patient had clinical proteinuria (> 300mg in 24-hour urine volume) after one and two years, and creatinine clearance was 62.6 ± 8.1 and 70.5 ± 0.7ml/min/1.73m2 at 12 and 24 months, respectively. The mean lower levels for tacrolimus at 1, 6, 12 and 24 months were 13.2, 10.3, 7.3 and 5.5ng/ml, respectively.
Histopathological findings
Ten kidney graft biopsies were performed in six patients, all of which were indicated to diagnose deteriorating kidney graft function an average of 6.1 months after the transplant (only the patient in pair 1 received a biopsy for prognostic information). Pathology reports concluded that these were due to two episodes of acute cellular rejection type 1A and 1B; seven biopsies showed no findings or minimum findings of interstitial fibrosis and/or tubular atrophy, and one showed signs of anticalcineurin toxicity (the one performed the latest, at 23 months after the transplant). All of these showed peritubular capillary C4d positivity, which was focal in eight biopsies and diffuse in two.
Associated complications and other adverse effects
During the follow-up period, nine patients had an infectious complication; the most common was urinary infection, which accounted for six episodes. All of the urinary infections occurred in the period immediately after the transplant, and the culprit agents were heterogeneous; Escherichia coli, present on two occasions, was the only repeated microbe. Citrobacter freundii, Coagulase-negative Staphylococcus, Enterococcus faecium, Pseudomonas aeruginosa, Enterobacter cloacae and Klebsiella pneumoniae were the other infectious agents found in diagnostic urinary cultures. Two episodes were polymicrobial.
A cytomegalovirus (CMV) infection was diagnosed in a CMV positive IgG donor/CMV positive IgG recipient. It was diagnosed due to positive antigenemia and blood PCR in a recipient who had experienced acute rejection and received a methylprednisolone bolus and an increase dose of immunosuppressants; the patient had received post-transplant prophylaxis lasting one month with oral valgancyclovir adjusted by kidney graft function. Urine immunocytochemistry was also positive for SV40 and for nuclear expression of polyoma virus (PMV) antigen in the immunohistochemistry for the same sample; however, there was no evidence of kidney disease due to PMV, since the immunohistochemical SV40 test was negative in the histological graft tissue biopsy.
There was a cutaneous herpes simplex infection at the surgical wound immediately after the transplant, and meningitis due to varicella zoster virus diagnosed with a cerebrospinal fluid PCR 25 months after the transplant.
On a vascular level, we only recorded the death of one patient three days after the transplant, which was due to dehiscence of vascular sutures made over a Dacron prosthesis from a previous aorto-bifemoral bypass. No other vascular problems were seen, and there were no episodes of thrombosis.
There was no haemolytic anaemia related to circulating isoagglutinins, nor were there cases of post-transplant lymphoproliferative disease or other malignancies.
DISCUSSION
In recent years, new organ sources are being used in the attempt to deal with the lack of sufficient brain dead donors needed to reduce the large number of patients on the waiting list. Barriers are being broken, such as transplants with a positive crossmatch and transplants with ABO incompatibility.5,25 In the experience of Tanabe20 and Ichimura’s19 Japanese group, Montgomery5 and Gloor’s6 group in the USA, and Tyden’s Swedish group,15-17,26 satisfactory results in the ABO-incompatible programme can be obtained on par with those in the ABO-compatible group, following different treatment strategies (ASI instead of plasma Exchange,5,6 RTX instead of splenectomy19-27). Other groups, such as Seller’s in Germany,28 Galliford’s in London29 or Oettle’s in Switzerland30 have begun successful ABO-incompatible transplant programmes. In Spain, no ABO-incompatible transplants were performed until 2006. The use of a desensitisation protocol based on ASI, IVIG and RTX was chosen as the most appropriate based on the Tyden’s group’s experience15,17 and the similar characteristics of the potential recipients of this type of transplant. We began with a protocol that has undergone modifications as we gain experience, due to the need to adjust the number of ASI sessions to the isoagglutinin titres on an individual basis in order to reach the correct titre level for performing the transplant (figure 1). The IVIG treatment was also divided into two doses administered on day -1 and day -4, and the RTX dose is currently given on day -9. With this desensitisation protocol, we have maintained very low post-transplant isoagglutinin titres that have not required administration of any additional ASI sessions later than 2 weeks after the transplant, as is published in the literature.26
Based on the original study by Alexandre,11 splenectomy is considered necessary in conjunction with the elimination of isoagglutinins with a plasma exchange or immunoadsorption technique,9,31 considering the leading role that the spleen plays in producing antibodies.19 Splenectomy has also been used to treat severe acute humoral rejection.32 The recent appearance of RTX as both an induction treatment15,17,27 and as a treatment for acute rejection mediated by antibodies33,34 has been suggested as a means to “pharmacological” splenectomy.31 A single dose is sufficient for eliminating peripheral B lymphocytes for several months, even with a dose of 375mg/m2 more than two years after the transplant,35 although most of the current protocols call for administering one or two doses of 100mg/m2 pre-transplant.19 When using RTX, we therefore obtain results that are similar to or even better than those achieved with splenectomy; it decreases the infection incidence rate, and does not require specific post-transplant anticoagulation, as Ichimaru and Takahara have confirmed.26 RTX has been used in our centre since its inception with good results, no need for post-transplant splenectomy and with no signs of a thrombosis episode. We are using a dosage of 375mg/m2 administered in a single dose at nine days prior to transplant, although as mentioned previously, we are assessing whether a lower dosage would be sufficient.
The use of RTX has also been beneficial compared to splenectomy with regard to the prevalence of post-transplant infections. In our series, we have a high infection rate, although nearly all of them were urinary infections occurring immediately after the transplant. The results are similar to those published by our group performing live-donor blood-type compatible transplants16,24,26 and cadaver transplants,36,37 and to results published by Galliford’s group,29 where there were eight cases of urinary sepsis and one surgical wound infection. Unlike in other groups where there were no CMV infections,29,30 our series contained one case, and so did Genberg’s group,16 in a patient who had experienced acute rejection and whose immunosuppressants were increased. The results for all of these groups are different from Tanabe’s group’s results, which included a 54% infection rate and three cases of CMV, although all responded to antiviral treatment based on intravenous gancyclovir.20 In our centre, we follow anti-CMV prophylaxis recommendations based on the serological state of the donor and recipient, and the induction immunosuppressant treatment used. With respect to PMV, we have only had one viral replication that tested positive by SV40 immunocytochemistry and by nuclear expression of polyoma virus (PMV) in urine, with no histological data indicating PMV-induced kidney disease in the immunohistochemistry. These data are similar to those from Oettl’s group,30 which recorded seven PMV replications that did not cause kidney disease, and also to Genberg’s group;16 in Galliford’s series,29 however, a PMV-induced case of kidney disease occurred after five months in a patient who had been treated for acute rejection and who did respond to reducing the immunosuppressants.
Although patient and graft survival rates are excellent, one death did occur resulting from a surgical complication. The acute rejection rate the acute rejection rate was 18.2%, (2 patients), which in this moment could be considered high.22,38-40 Considering that the transplant number is low and represents a very specific population with a high immunological risk, we feel that this is acceptable; one episode of type IA cellular rejection occurred in a hypersensitized patient with a pretransplant positive crossmatch, and the other episode was in a sensitized patient. These patients responded correctly to specific anti-rejection treatment. The data obtained from renal graft biopsies (although protocol biopsies were not routinely performed in our centre at that time) are very satisfactory. Eighty percent of the biopsies are normal or have minimal interstitial fibrosis and/or tubular atrophy. These results resemble those published for routine biopsies,30 but the biopsies are performed at a later time (6.1 months) since they are indicated in cases of abnormal kidney graft function or not reaching the function that was previously anticipated. We must point out that 100% of the biopsies show positive peritubular capillary C4d immunostaining; 80% of these are focal, and one of the patients in which it was diffuse experienced acute cellular rejection. The same results were published by Oettl; 100% of the biopsies had a positive C4d staining in a total of 46 routine biopsies in eight patients (up to 18 months post-transplant); however, the staining was diffuse except for one case that was focal, and they did not meet the Banff criteria for acute or chronic antibody-mediated rejection.24 One special case is that of patients with a longer follow-up time who are C4d positive, but still do not meet rejection criteria and who have stable creatinine and proteinuria values and no variations in creatinine clearance that would suggest that peritubular capillary C4d is a biomarker for chronic rejection, unlike that stated by Montgomery this year.41
The medium-term kidney graft function for these patients, expressed in plasma creatinine, can be considered excellent if compared with results from blood-type compatible live-donor transplants16,24,26 and cadaver transplants.36,37 The results are comparable to those published by Tyden et al (26) for the ABO compatible live-donor transplants: at one year 1.16 ± 0.56 mg/dl in our data vs. 124.4 µmol/l (1.41 mg/dL) in Tyden’s group,26 and a longer than those from deceased donors (1.4mg/dl in Matas’ group36 and 1.53mg/dl for published data in our group37).
In short, we feel that ABO-incompatible live-donor kidney transplants can join the array of transplant techniques at our centre, thus increasing the number of live-donor transplants while ensuring results comparable to those of compatible blood-type live-donor transplants and better than for cadaver-donor kidney transplants. However, we require more information with a larger number of patients and better follow-up in order to evaluate long-term results.
To conclude, our experience shows ABO-incompatible kidney transplant to be an effective and safe alternative in cases in which a live-donor option is possible.
Figure 1.
Table 1. Donor-recipient characteristics, with immunosuppressant regimen and follow-up time
Table 2. ABO characteristics of donor and recipient. Use of specific anti-ABO desensitisation protocol
Figure 2.
Figure 3.