Post-transplant proteinuria is associated with lower graft and patient survival. Renin–angiotensin–aldosterone system blockers are used to reduce proteinuria and improve renal outcome. Although it is known that a high salt intake blunts the antiproteinuric effect of ACEI and ARB drugs in non-transplant patients, this effect has not been studied in kidney transplant recipients.
ObjectiveTo analyse the relationship between sodium intake and the antiproteinuric effect of ACEI/ARB drugs in kidney transplant recipients.
MethodsWe selected 103 kidney transplant recipients receiving ACEI/ARB drugs for more than 6 months due to proteinuria >1g/day. Proteinuria was analysed at baseline and at 6 months after starting ACEI/ARB treatment. Salt intake was estimated by urinary sodium to creatinine ratio (uNa/Cr).
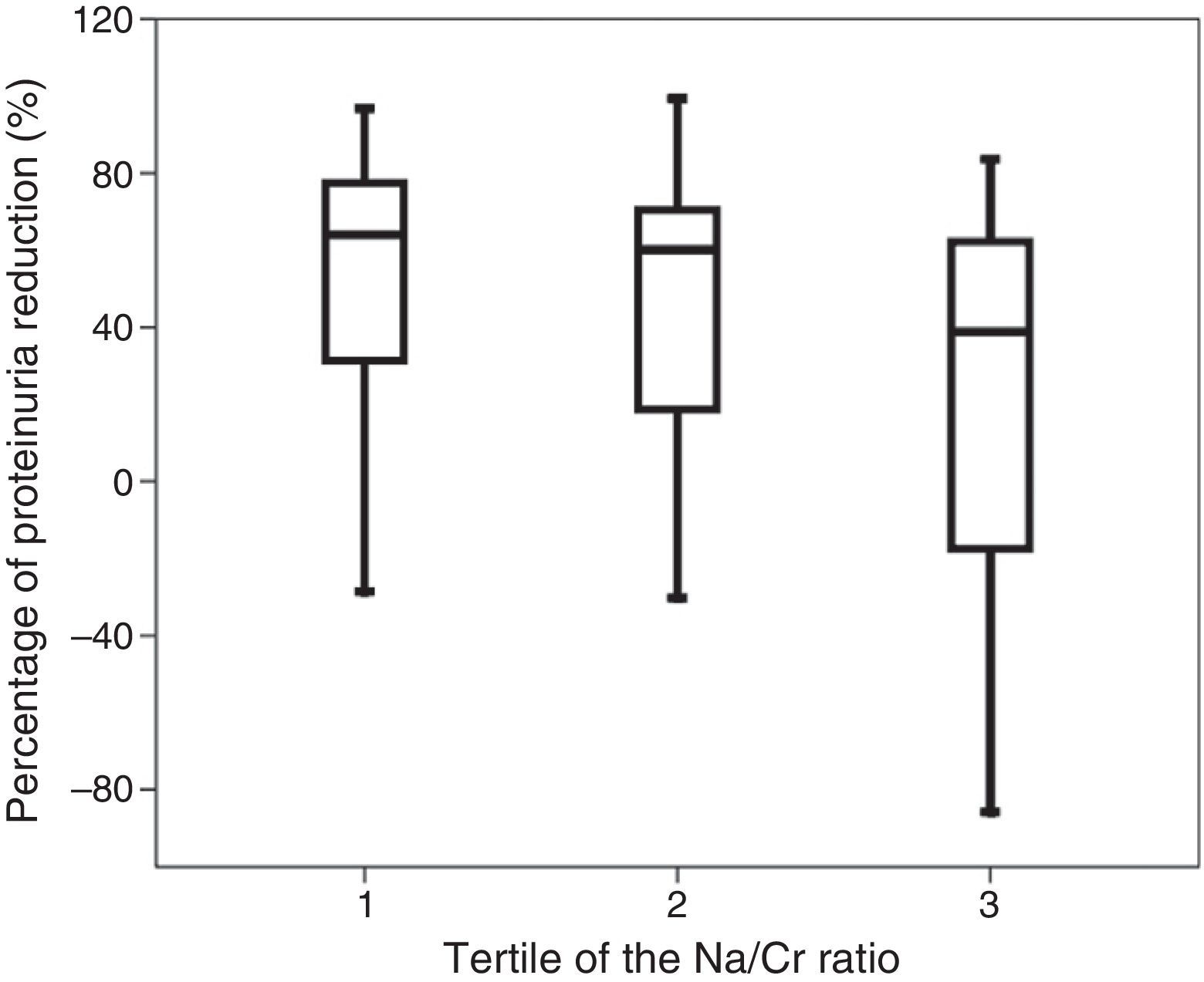
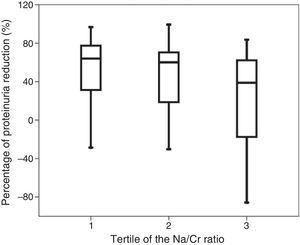
ResultsProteinuria fell to less than 1g/day in 46 patients (44.7%). High uNa/Cr was associated with a smaller proteinuria decrease (r=−0.251, p=0.011). The percentage of proteinuria reduction was significantly lower in patients in the highest uNa/Cr tertile [63.9% (IQR 47.1%), 60.1% (IQR 55.4%), 38.9% (IQR 85.5%), p=0.047]. High uNa/Cr independently relates (OR 2.406 per 100mEq/g, 95% CI: 1.008–5.745, p=0.048) to an antiproteinuric response <50% after renin–angiotensin–aldosterone system blockade.
ConclusionsA high salt intake results in a smaller proteinuria decrease in kidney transplant recipients with proteinuria treated with ACEI/ARB drugs.
La proteinuria postrasplante renal se asocia a una disminución en la supervivencia del injerto y del paciente. Para reducir la proteinuria y mejorar el pronóstico renal se recomienda asociar fármacos bloqueantes del sistema renina-angiotensina-aldosterona (RAA). Aunque en los pacientes no trasplantados se ha demostrado que la dieta rica en sal reduce el efecto antiproteinúrico de los IECA y ARA-II, este efecto no se ha estudiado en los trasplantados renales.
ObjetivoValorar la relación entre la ingesta de sodio y el efecto antiproteinúrico de los IECA/ARA-II en los trasplantados renales.
MétodosSeleccionamos a 103 trasplantados tratados con IECA/ARA-II más de 6 meses por proteinuria > 1 g/día. La proteinuria se analizó al inicio del tratamiento y a los 6 meses. La ingesta de sal se estimó con el cociente urinario sodio/creatinina (uNa/Cr).
ResultadosEn 46 pacientes (44,7%) la proteinuria disminuyó < 1 g/día. Un uNa/Cr elevado se relaciona con un menor descenso de la proteinuria (r = −0,251; p = 0,011). El porcentaje de reducción de la proteinuria fue significativamente menor en los pacientes en el tercil más alto de uNa/Cr [63,9% (RIC 47,1%); 60,1% (RIC 55,4%); 38,9% (RIC 85,5%); p = 0,047]. Un uNa/Cr elevado se relaciona de forma independiente (OR 2,406 por 100 mEq/g; IC 95%: 1,008–5,745; p = 0,048) a una respuesta antiproteinúrica < 50% tras el bloqueo del eje RAA.
ConclusionesEn los trasplantados renales con proteinuria tratados con IECA/ARA-II una ingesta elevada de sal se asocia con un menor descenso de la proteinuria.
The development of proteinuria, even in a small amount, after kidney transplantation is associated with a reduction in graft and patient survival, and so the control of proteinuria is of clinical relevance.1–3 Unfortunately, a large number of kidney transplant recipients develop proteinuria. In a study of 613 kidney transplants, up to 45% had proteinuria of more than 150mg/day, and in 65% of these patients proteinuria was below 500mg/day. Biopsies from patients with proteinuria showed mainly interstitial fibrosis and tubular atrophy, or non-specific findings, except in those with proteinuria above 1500mg/day, in which glomerular involvement was predominant.4 The factors inducing the onset of proteinuria include: transplant from a female donor to a male recipient; advanced donor age; kidney function; blood pressure; cell rejection and antibody-mediated rejection; recurrence of glomerulonephritis; prolonged warm and cold ischaemia; and delayed initiation of graft function.4–6
The measures currently used to reduce post-transplant proteinuria include strict control of blood pressure, renin–angiotensin–aldosterone system (RAAS) blockade with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor II blockers (ARBs), lipid control, stop smoking and maintaining a healthy weight.7 Specifically, the KDIGO guidelines recommend using ACE inhibitors or ARBs in patients with recurrent glomerulonephritis and proteinuria and in hypertensive patients with proteinuria ≥1g/day.8
In the general population, RAAS blockade has been shown to be effective in reducing proteinuria, controlling hypertension and reducing the progression of chronic kidney disease (CKD) in patients with diabetic and non-diabetic nephropathy.9,10 Although some studies have demonstrated the efficacy of the antiproteinuric effect of RAAS blockade in kidney transplantation,11,12 there is no precise information on the efficacy of proteinuria reduction on preservation of renal function and improvement of graft and patient survival.12–16
In non-transplant CKD patients, several factors can reduce the antiproteinuric effect of RAAS blockade.17–22 Salt intake is one of these factors. One meta-analysis that included 11 studies was able to quantify that albuminuria was decreased by 32.1% for every reduction in sodium intake of 92mEq/day.22 None of the cohorts considered in the meta-analysis included kidney transplant patients. The aim of our study was to assess the relationship between sodium intake and the antiproteinuric effect of ACE inhibitors and ARBs in our population of kidney transplant patients.
MethodsStudy population and designWe selected 137 patients from a population of 1423 kidney transplants performed at our site between October 1986 and May 2012. Patients included were those: (1) who have been transplanted for more than 3 months (2) had proteinuria greater than 1g/day; (3) who had been treated with ACE inhibitors or ARBs for more than 6 months; and (4) had a urinary sodium/creatinine ratio value available before starting treatment with ACE inhibitors or ARBs. We excluded 34 patients with changes in plasma creatinine greater than 25% before starting ACE inhibitors or ARBs, and patients who had modified their diuretic therapy in the month before starting ACE inhibitors or ARBs or over the 6 months of follow-up. In our unit, all patients are trained to perform an accurate collection of a 24-h urine protein test in each routine visit, and all receive specific dietary advice to follow a diet low in fat and salt. A total of 40 patients (38.8%) were on diuretic therapy. The doses of ACE inhibitors and ARBs were standardised for an equivalent dose of enalapril according to previous drug guidelines and studies on the antiproteinuric effect with equivalent doses.23–25 The study was conducted in accordance with the criteria set forth in the Declaration of Helsinki.
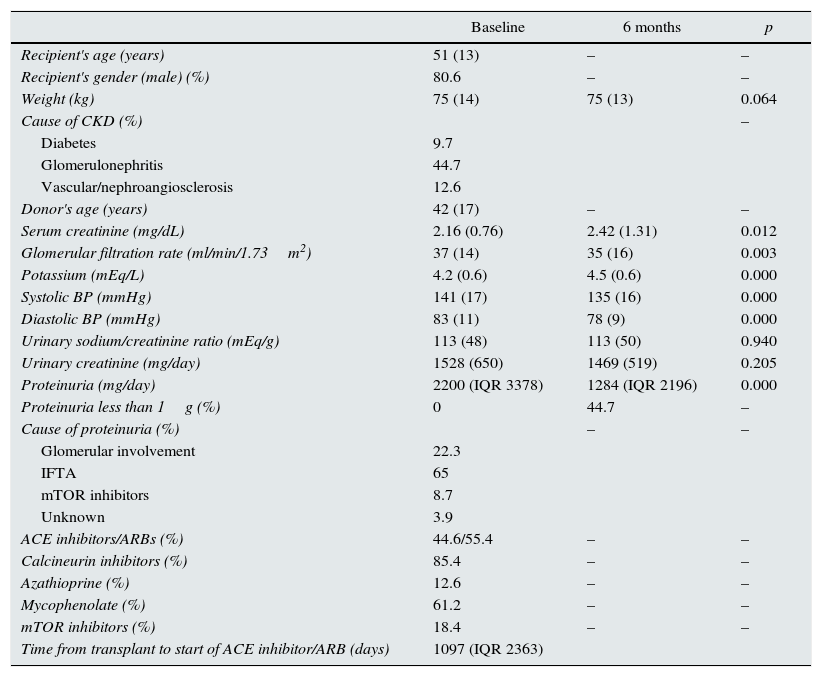
Data collectionThe patient characteristics and laboratory data were collected at the beginning of the RAAS blockade and at 6 months (Table 1), and they were extracted prospectively from the database of kidney transplant patients from our site. The variables collected were ages of the donor and recipient, weight, cause of CKD, cause of proteinuria, immunosuppressive drugs, time since transplant at the beginning of the RAAS blockade and laboratory data. Proteinuria was determined by the pyrogallol red-molybdate (PRM) method for the 24-h urine protein test. Sodium intake was estimated by 24-h urinary sodium excretion and normalised with creatinine with the sodium/creatinine ratio to avoid possible errors in the collection. Blood pressure was measured 3 times, separated by 5min by an automatic system, and the final reading was used.
Patient characteristics at baseline and at 6 months.
| Baseline | 6 months | p | |
|---|---|---|---|
| Recipient's age (years) | 51 (13) | – | – |
| Recipient's gender (male) (%) | 80.6 | – | – |
| Weight (kg) | 75 (14) | 75 (13) | 0.064 |
| Cause of CKD (%) | – | ||
| Diabetes | 9.7 | ||
| Glomerulonephritis | 44.7 | ||
| Vascular/nephroangiosclerosis | 12.6 | ||
| Donor's age (years) | 42 (17) | – | – |
| Serum creatinine (mg/dL) | 2.16 (0.76) | 2.42 (1.31) | 0.012 |
| Glomerular filtration rate (ml/min/1.73m2) | 37 (14) | 35 (16) | 0.003 |
| Potassium (mEq/L) | 4.2 (0.6) | 4.5 (0.6) | 0.000 |
| Systolic BP (mmHg) | 141 (17) | 135 (16) | 0.000 |
| Diastolic BP (mmHg) | 83 (11) | 78 (9) | 0.000 |
| Urinary sodium/creatinine ratio (mEq/g) | 113 (48) | 113 (50) | 0.940 |
| Urinary creatinine (mg/day) | 1528 (650) | 1469 (519) | 0.205 |
| Proteinuria (mg/day) | 2200 (IQR 3378) | 1284 (IQR 2196) | 0.000 |
| Proteinuria less than 1g (%) | 0 | 44.7 | – |
| Cause of proteinuria (%) | – | – | |
| Glomerular involvement | 22.3 | ||
| IFTA | 65 | ||
| mTOR inhibitors | 8.7 | ||
| Unknown | 3.9 | ||
| ACE inhibitors/ARBs (%) | 44.6/55.4 | – | – |
| Calcineurin inhibitors (%) | 85.4 | – | – |
| Azathioprine (%) | 12.6 | – | – |
| Mycophenolate (%) | 61.2 | – | – |
| mTOR inhibitors (%) | 18.4 | – | – |
| Time from transplant to start of ACE inhibitor/ARB (days) | 1097 (IQR 2363) |
ARBs: angiotensin receptor antagonists; CKD: chronic kidney disease; IFTA: interstitial fibrosis and tubular atrophy; ACE inhibitors: angiotensin-converting enzyme inhibitors; mTOR: mammalian target of rapamycin; IQR: interquartile range; BP: blood pressure.
The standard deviation is shown in parentheses.
Categorical variables were expressed as relative frequencies and continuous variables as the mean±standard deviation, except for those who had normal distribution, which were reported as median and interquartile range (IQR). Tertiles for the urinary sodium/creatinine ratio were established, and salt intake was estimated (<87mEq/g; between 87 and 138mEq/g; >138mEq/g; cut-offs of 87 and 138mEq/g are equivalent to 6.4 and 9.4g of salt per day). Proteinuria, urinary sodium/creatinine ratio, creatinine clearance in urine, kidney function and serum potassium were compared at baseline and at 6 months using the Student's t-test for paired samples. The relationship between quantitative variables was analysed by correlation analysis. The Kruskal–Wallis test was used to establish the relationship between the percentage of decrease in proteinuria and the tertiles of urinary sodium/creatinine ratio. Inadequate antiproteinuric response was defined as a percentage of reduction in proteinuria of less than 50%. The risk factors for inadequate antiproteinuric response were analysed using the Student's t-test, Mann–Whitney U test and Chi-squared test. A multivariate logistic regression analysis was conducted with all significant variables. A p-value <5% was considered statistically significant. SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) was used to perform the analysis.
ResultsPatient characteristics at baseline and at 6 months are shown in Table 1. In all, 55.4% of patients received ACE inhibitors and 44.6%, ARBs. Six months after starting the RAAS blockade, serum creatinine and potassium levels increased, systolic and diastolic BP decreased, and 24-h proteinuria was significantly reduced (Table 1). By contrast, there were no significant differences at 6 months in urinary sodium or creatinine elimination, nor in urinary sodium/creatinine ratio (Table 1). In 46 patients (44.7%) proteinuria was reduced below one gram after 6 months of RAAS blockade. We found no significant differences in the initial sodium/creatinine ratio amongst patients with and without diuretic treatment (p=0.083).
A high urinary sodium/creatinine ratio was associated with a smaller reduction in proteinuria at 6 months (r=−0.251; p=0.011) and a lower percentage of decreased proteinuria from baseline proteinuria (r=−0.211; p=0.033). Similarly, the percentage of reduction in proteinuria from baseline was significantly lower in patients in the highest tertile for the urinary sodium/creatinine ratio [63.9% (IQR 47.1%); 60.1% (IQR 55.4%); 38.9% (IQR 85.5%); p=0.047] (Fig. 1). The decrease in the absolute value for proteinuria at 6 months was lower for the highest tertile, but was not statistically significant [1161mg (IQR 2406mg); 978mg (IQR 1299mg); 696mg (IQR 1422mg); p=0.056].
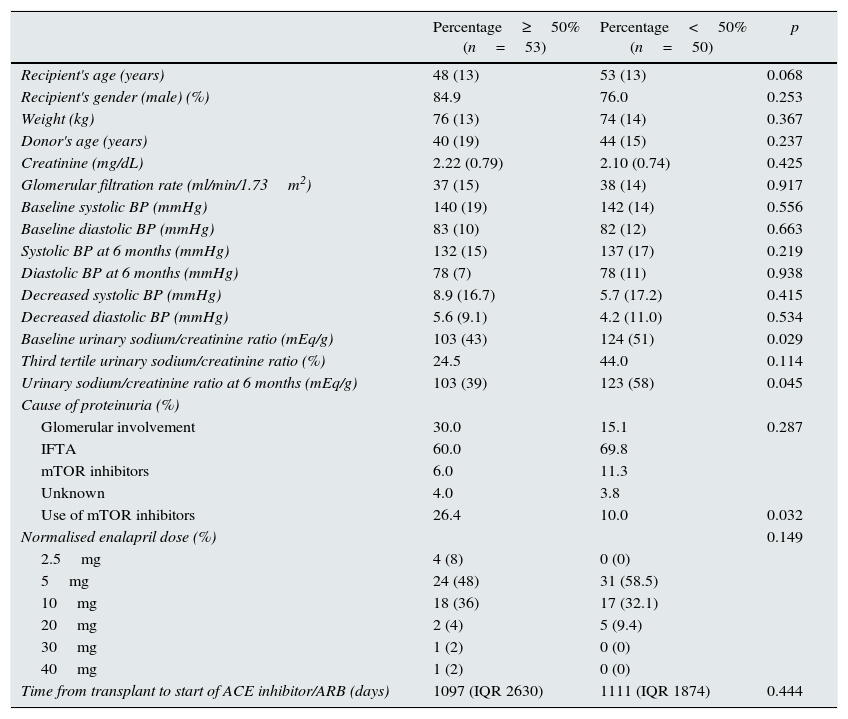
Table 2 shows the factors that are in relation with a percent reduction of in proteinuria of less than 50% (52 patients). The use of mTOR inhibitors and a lower urinary sodium/creatinine ratio was significantly related to a reduction in proteinuria greater than 50%. Neither serum creatinine nor glomerular filtration rate were associated with the percent decreased in proteinuria (Table 2) or reduction of proteinuria in absolute values (data not shown). The doses of ACE inhibitors and ARBs standardised for enalapril were not associated with the decreased in proteinuria (p=0.149). Using a logistic regression analysis, including the significant variables (use of mTOR inhibitors and sodium/creatinine ratio), the urinary sodium/creatinine ratio was the only factor related independently (OR 2.406 by 100mEq/g, 95% CI: 1.008–5.745; p=0.048) to an inadequate antiproteinuric response after RAAS blockade in kidney transplant recipients.
Risk factors related to a percentage decrease in proteinuria of greater than 50%.
| Percentage≥50% (n=53) | Percentage<50% (n=50) | p | |
|---|---|---|---|
| Recipient's age (years) | 48 (13) | 53 (13) | 0.068 |
| Recipient's gender (male) (%) | 84.9 | 76.0 | 0.253 |
| Weight (kg) | 76 (13) | 74 (14) | 0.367 |
| Donor's age (years) | 40 (19) | 44 (15) | 0.237 |
| Creatinine (mg/dL) | 2.22 (0.79) | 2.10 (0.74) | 0.425 |
| Glomerular filtration rate (ml/min/1.73m2) | 37 (15) | 38 (14) | 0.917 |
| Baseline systolic BP (mmHg) | 140 (19) | 142 (14) | 0.556 |
| Baseline diastolic BP (mmHg) | 83 (10) | 82 (12) | 0.663 |
| Systolic BP at 6 months (mmHg) | 132 (15) | 137 (17) | 0.219 |
| Diastolic BP at 6 months (mmHg) | 78 (7) | 78 (11) | 0.938 |
| Decreased systolic BP (mmHg) | 8.9 (16.7) | 5.7 (17.2) | 0.415 |
| Decreased diastolic BP (mmHg) | 5.6 (9.1) | 4.2 (11.0) | 0.534 |
| Baseline urinary sodium/creatinine ratio (mEq/g) | 103 (43) | 124 (51) | 0.029 |
| Third tertile urinary sodium/creatinine ratio (%) | 24.5 | 44.0 | 0.114 |
| Urinary sodium/creatinine ratio at 6 months (mEq/g) | 103 (39) | 123 (58) | 0.045 |
| Cause of proteinuria (%) | |||
| Glomerular involvement | 30.0 | 15.1 | 0.287 |
| IFTA | 60.0 | 69.8 | |
| mTOR inhibitors | 6.0 | 11.3 | |
| Unknown | 4.0 | 3.8 | |
| Use of mTOR inhibitors | 26.4 | 10.0 | 0.032 |
| Normalised enalapril dose (%) | 0.149 | ||
| 2.5mg | 4 (8) | 0 (0) | |
| 5mg | 24 (48) | 31 (58.5) | |
| 10mg | 18 (36) | 17 (32.1) | |
| 20mg | 2 (4) | 5 (9.4) | |
| 30mg | 1 (2) | 0 (0) | |
| 40mg | 1 (2) | 0 (0) | |
| Time from transplant to start of ACE inhibitor/ARB (days) | 1097 (IQR 2630) | 1111 (IQR 1874) | 0.444 |
ARBs: angiotensin receptor antagonists; IFTA: interstitial fibrosis and tubular atrophy; ACE inhibitors: angiotensin-converting enzyme inhibitors; mTOR: mammalian target of rapamycin; IQR: interquartile range; BP: blood pressure.
The standard deviation is shown in parentheses.
As in previous studies in transplant and non-transplant patients,9,26 we have observed that RAAS blockers significantly reduce proteinuria. After 6 months of treatment, mean proteinuria was reduced from 3.6 to 2.2g/day, and in almost half of patients (44.7%) proteinuria fell below 1g/day. The advantages of the use of ACE inhibitors/ARBs in kidney transplants remain controversial, with studies where there is no histological improvement, and no change in graft or patient survival, or in the cardiovascular risk15,16,27; others studies show an increase in patient survival, but not in the graft survival28; and there are also studies showing an improvement in both graft and patient survival.29 In this latter study, the greatest benefit on graft outcome was observed in patients with higher proteinuria.29 Whether due to a specific effect of RAAS blockade, or not, what is clear is that the reduction of short-term albuminuria has a long-term nephroprotective effect.
In a meta-analysis of 21 clinical trials that included 78,342 patients, Heerspink et al. observed that for every 30% reduction in albuminuria achieved with RAAS blockade, the risk to reach stage 5 CKD was reduced by 32% in the long term analysis.30 The reduction of approximately 39% in proteinuria observed in our study with RAAS blockers may lead to a reduction in the loss of long-term renal grafts of more than 30%, although this effect should be evaluated in long-term studies. In contrast to the general population, in the kidney transplant population the reduction of proteinuria with ACE inhibitors/ARBs has not been demonstrated to be reduce the progression of renal disease. This may be due to the characteristics of the transplant patients (i.e. immunosuppressive therapy, especially with calcineurin inhibitors and alloimmune damage) as well as to methodological problems. For example, in the meta-analysis by Heerspink et al., a sample size of less than 1250 patient or the cause of the nephropathy affected the ability of the studies to show any benefit from RAAS blockade.30 In kidney transplantation it is difficult to conduct long-term studies with large numbers of patients enough to demonstrate the long-term benefit of ACE inhibitors and ARBs.
The effect of low-salt diet on BP is recognised both in the transplant population as well as in the general population.31 However, many patients with CKD have a salt intake above the WHO recommendations (below 5g/day).32 Amongst kidney transplant recipients, up to 87% have a urinary sodium excretion that reflects a salt intake above 5g. In addition, salt intake is maintained over time in each patient and in the overall population, despite the recommendations made.31,33,34 This suggests that, despite some difficulties, we have an opportunity to improve outcomes in kidney transplant recipients by intensifying dietary measures without requiring drug.
The main finding of our study is that a high-salt diet limits the antiproteinuric effect of ACE inhibitors and ARBs in the kidney transplant population, similar to previously published studies of CKD patients. In a prospective study of 500 non-diabetic patients with CKD, Vegter et al. showed that patients in the highest tertile of urinary sodium/creatinine ratio showed a minor decrease in proteinuria with ramipril (20%) compared with patients with lower salt intake (25% and 31%), leading to an increased risk of developing stage 5 CKD.17 In our kidney transplant patients the percent reduction in proteinuria in the different tertiles of sodium/creatinine ratio (from to the highest to the lowest) was 16%, 41% and 43%, respectively. The antiproteinuric effect of low-salt diet has been proven to be more effective even than double RAAS blockade, or as effective as diuretic therapy.18,20 Thus, in a randomised study of 52 patients with non-diabetic nephropathy, the reduction in proteinuria caused by adding a low-salt diet to treatment with ACE inhibitors was significantly greater than the reduction of proteinuria by adding ARBs to ACE inhibitors (51% vs. 21%; p<0.001).18 Also in 34 patients with proteinuria without diabetes, in whom treatment with losartan decreased proteinuria by 30%, the addition of hydrochlorothiazide achieved a reduction of 56%, and of 55% by adding a low-salt diet.20
Therefore, it seems advisable to insist that the transplant patients also must follow a diet low in salt, especially in those with proteinuria who have not responded, or responded only partially, with RAAS blockade. Given the differences between the highest tertile for salt intake and the middle and lower tertiles in our study, we recommend at least a moderate decrease in sodium intake to improve the antiproteinuric response to treatment with ACE inhibitors/ARBs, without requiring a severe restriction. It is foreseeable that, in parallel to that observed in the non-transplant CKD population, a greater reduction in proteinuria will contribute to improving renal graft survival.
The pathophysiological mechanisms that contribute to the antiproteinuric effect of salt restriction are not fully known. In normal conditions, salt intake is associated with an increase in serum sodium that triggers the thirst centre and stimulates the secretion of antidiuretic hormone. All this leads to increased blood volume, blood pressure and glomerular filtration rate. This decreases the activating stimulus of renin, which makes RAAS blockade less effective for hypertension and proteinuria. If serum sodium decreases, blood volume and blood pressure are also reduced; the RAAS blockade is activated and becomes more effective.22 Furthermore, sodium appears to play a role through the endothelium. Verhave et al. showed that salt intake increases albuminuria independently of blood pressure,35 a fact that reflects the specific endothelial damage. We also found a relationship between changes in blood pressure and antiproteinuric response, a finding previously described by some authors and which suggests that the reduction in proteinuria induced by salt restriction is influenced both by intraglomerular pressure and systemic BP.17 This endothelial damage could also be mediated by inflammatory mechanisms: it has even been observed that high salt intake is associated with higher albuminuria and with elevated CRP in hypertensive patients.22,36
Analysing the factors that could be related to the antiproteinuric response, we noted that the use of mTOR inhibitors is associated with a greater antiproteinuric response to ACE inhibitors/ARBs, although this finding was not confirmed in the multivariate analysis. The use of ACE inhibitors/ARBs has been shown to be effective in the literature in treating and even preventing proteinuria that develops after conversion from calcineurin inhibitors to mTOR inhibitors.37 Several mechanisms have been described that contribute to the onset of proteinuria with mTOR inhibitors. Most times proteinuria develops after discontinuation of calcineurin inhibitors, probably related to the interruption of their vasoconstrictor effect.38,39 There are some assumptions that point to possible direct glomerular and tubular damage and there have been reported cases of focal and segmental glomerulosclerosis.39,40 There are no specific studies demonstrating a better antiproteinuric response with ACE inhibitors/ARBs in patients treated with mTOR inhibitors. To explain our findings, we can speculate with 2 potential mechanisms: (1) ACE inhibitors and ARBs reduce glomerular capillary pressure that would increase after the withdrawal of calcineurin inhibitors and beginning the mTOR inhibitor; and 92) both ACE inhibitors and ARBs reverse the damage mediated by angiotensin II, specifically induced by mTOR on albumin uptake in the proximal tubule.40
The main limitation of our study is that it is an observational study in which patients are on different drugs and different ACE inhibitors and ARBs. However, it does reflect routine clinical practice in that the doses are adjusted according to potassium, creatinine and BP. There were no different antiproteinuric responses seen when normalising to a standard dose of enalapril, which can be explained because most patients were on 5mg of enalapril and the same dose was maintained throughout the study period (Table 2). As discussed above, the absence of a relationship between changes in BP and the antiproteinuric effect of salt restriction indicates that low-salt diet exerts its additional antiproteinuric effect through partially independent mechanisms of lower BP and doses of ACE inhibitors/ARBs. A second limitation is that this is a single-centre study, with a limited sample size. However, the significant relationship between urinary sodium/creatinine ratio and antiproteinuric response indicates that there is an association between them. There need to be larger, multicentre, prospective studies to confirm the role of sodium intake in the antiproteinuric response to RAAS blockade in renal transplant patients.
In conclusion, our study indicates that, in renal transplant patients with proteinuria treated with ACE inhibitors/ARBs, high salt intake is associated with a smaller decrease in proteinuria and possibly with lower graft survival. Avoiding excessive salt intake is a necessary and effective measure that should be combined with RAAS blockade.
Conflicts of interestThe authors have no conflicts of interest.
Please cite this article as: Monfá E, Rodrigo E, Belmar L, Sango C, Moussa F, Ruiz San Millán JC, et al. La ingesta elevada de sodio disminuye la respuesta antiproteinúrica del bloqueo del eje renina-angiotensina-aldosterona en el trasplante renal. Nefrología. 2016;36:545–551.