Topiroxostat, an inhibitor of xanthine oxidoreductase (XOR) was shown to reduce urinary albumin excretion of hyperuricemic patients with chronic kidney disease. However, its pharmacological mechanism is not well understood. In this study, we examined the effects of topiroxostat on glomerular podocytes. Podocyte is characterized by foot process and a unique cell-cell junction slit diaphragm functioning as a final barrier to prevent proteinuria.
MethodsThe effects of topiroxostat on the expressions of podocyte functional molecules were analysed in db/db mice, a diabetic nephropathy model, anti-nephrin antibody-induced rat podocyte injury model and cultured podocytes treated with adriamycin.
ResultsTopiroxostat treatment ameliorated albuminuria in db/db mice. The expression of desmin, a podocyte injury marker was increased, and nephrin and podocin, key molecules of slit diaphragm, and podoplanin, an essential molecule in maintaining foot process were downregulated in db/db mice. Topiroxostat treatment prevented the alterations in the expressions of these molecules in db/db mice. XOR activity in kidney was increased in rats with anti-nephrin antibody-induced podocyte injury. Topiroxostat treatment reduced XOR activity and restored the decreased expression of nephrin, podocin and podoplanin in the podocyte injury. Furthermore, topiroxostat enhanced the expression of podoplanin in injured human cultured podocytes.
ConclusionsPodocyte injury was evident in db/db mice. Topiroxostat ameliorated albuminuria in diabetic nephropathy model by preventing podocyte injury. Increase of XOR activity in kidney contributes to development of podocyte injury caused by stimulation to slit diaphragm. Topiroxostat has an effect to stabilize slit diaphragm and foot processes by inhibiting the reduction of nephrin, podocin and podoplanin.
El topiroxostat, un inhibidor de la xantina oxidorreductasa (XOR), mostró reducir la excreción de albúmina en la orina de pacientes hiperuricémicos con enfermedad renal crónica. Sin embargo, su mecanismo farmacológico no se conoce con exactitud. En este estudio examinamos los efectos del topiroxostat en los podocitos glomerulares. El podocito se caracteriza por unas prolongaciones en forma de pie y un diafragma de hendidura de unión célula-célula único que funciona como barrera final en la prevención de la proteinuria.
MétodosSe analizaron los efectos del topiroxostat en las expresiones de las moléculas funcionales de los podocitos en ratones db/db, en un modelo de nefropatía diabética, en un modelo de lesión podocitaria inducida por anticuerpos antinefrina en ratas y en podocitos cultivados tratados con adriamicina.
ResultadosEl tratamiento con topiroxostat mejoró la albuminuria en ratones db/db. La expresión de la desmina, un marcador de lesión podocitaria, estaba aumentada, y la nefrina y la podocina, moléculas clave del diafragma de hendidura, y la podoplanina, una molécula esencial en el mantenimiento de las prolongaciones en forma de pie, estaban atenuadas en los ratones db/db. El tratamiento con topiroxostat evitó alteraciones en las expresiones de estas moléculas en los ratones db/db. La actividad de la XOR en el riñón se incrementó en ratas con lesión podocitaria inducida por anticuerpos antinefrina. El tratamiento con topiroxostat redujo la actividad de la XOR y restauró la disminución de la expresión de nefrina, podocina y podoplanina en la lesión podocitaria. Además, el topiroxostat aumentó la expresión de podoplanina en podocitos humanos cultivados lesionados.
ConclusionesLa lesión podocitaria era evidente en ratones db/db. El topiroxostat mejoró la albuminuria en el modelo de nefropatía diabética al prevenir la lesión podocitaria. El aumento de la actividad de la XOR en el riñón contribuye al desarrollo de la lesión podocitaria causada por la estimulación del diafragma de hendidura. El topiroxostat tiene un efecto de estabilización del diafragma de hendidura y de las prolongaciones en forma de pie al inhibir la reducción de nefrina, podocina y podoplanina.
Recent epidemiological studies indicate a significant correlation between hyperuricemia and chronic kidney disease (CKD). It is reported that elevated serum uric acid (UA) increases the risk for the development of new-onset kidney disease,1 and is an independent predictor for end-stage renal disease.2,3 Some clinical and experimental studies suggested that high serum UA level contributes to the development of diabetic nephropathy, one of the most prominent primary diseases of CKD.4–6 Topiroxostat is a xanthine oxidoreductase (XOR) inhibitor developed for treatment and management of hyperuricemia and gout. It is reported that topiroxostat reduced proteinuria in hyperuricemic patients with CKD.7–9 The experimental studies showed that topiroxostat ameliorated proteinuria in several nephrotic models.10–13 However, the precise pharmacological mechanisms of topiroxostat on proteinuria remained unclear.
It is now accepted that proteinuria of several types of kidney diseases resulted from injury of visceral glomerular epithelial cell (podocyte).14 Podocyte is a highly specialized terminally differentiated cell characterized by interdigitating foot processes and a slit diaphragm connecting adjacent foot processes. The slit diaphragm is a unique cell-cell junction of podocyte and functions as a final barrier preventing the leak of plasma proteins into primary urine.15
In this study, we analysed the effect of topiroxostat on the expressions of desmin, a marker of podocyte injury16 and podocyte functional molecules such as nephrin, podocin and podoplanin in db/db mice, a diabetic nephropathy model and anti-slit diaphragm antibody-induced rat model.17,18 Then, we analysed the direct effect of topiroxostat on podocyte with cultured podocytes. Our findings indicated that topiroxostat has a direct effect on podocyte and ameliorated proteinuria by inhibiting the reduction of podocyte functional molecules such as nephrin, podocin and podoplanin.
MethodsChemicalsTopiroxostat was synthesized by Tateyama Kasei Co., Ltd., Toyama, Japan. Adriamycin was purchased from Kyowa Hakko Kirin, Tokyo, Japan.
Animal studiesAll animal experiments with mice and rats were approved by the committees on animal care of Sanwa Kagaku Kenkyusho and Niigata University School of Medicine, respectively.
Studies with db/db mice: Male db/db and littermate db/lean mice (db/m) were obtained from Charles River Japan (Osaka, Japan). Twenty db/db mice (diabetic mice) and 14db/m mice (normal mice) were used. Twenty four-hr urine samples of the mice were collected before the start of the study. The mice received topiroxostat-containing food (3mg/kg BW/day) or vehicle food for 4 weeks (9–13 weeks of age). At 13 weeks of age, the mice were sacrificed and kidneys and blood samples were obtained. Twenty four-hr urine samples were collected just before sacrifice. Kidney materials were used for immunofluorescence (IF) and real-time PCR analyses.
Studies with rat podocyte injury model caused by anti-nephrin antibody: Female Wistar rats were purchased from Charles River Japan (Atsugi, Japan). Twelve rats were injected with anti-nephrin antibody (8mg/head). Six rats each were orally administered with topiroxostat (1mg/kg BW/day) or vehicle (DW) daily from 2 days before the antibody injection. As a normal control, four other rats were injected with PBS and orally administered with DW. The rats were sacrificed on day 5 after the antibody injection. After blood sampling, the kidneys were removed. Kidney samples were used for immunofluorescence (IF) studies and biochemical analyses.
Studies with cultured podocyteA conditionally immortalized human podocyte cell line was kindly donated by Dr. Saleem (Bristol Medical School, Bristol, United Kingdom). Cultivation of the cell line was conducted as described previously.19 Differentiated cultured podocytes were incubated with adriamycin (2μg/ml) or vehicle (PBS) for 72h. The cells were harvested and used for real-time PCR to analyse the expression of podocyte functional molecules. Then, to analyse the effect of topiroxostat, the cells were treated with topiroxostat (25μg/ml) 1h before the treatment with adriamycin. The cells were harvested 72h after the adriamycin treatment and used for real-time PCR.
Biochemical analysisUrinary albumin was measured using an enzyme-linked immunosorbent assay Kit (Bethyl Laboratory, AL, USA). Blood urea nitrogen (BUN) was measured with CII test Wako UN (Wako Pure Chemical Industries, Osaka, Japan). HbA1c was determined with high performance liquid chromatography (Tosoh, Tokyo, Japan). Plasma levels of XOR activity, hypoxanthine (HX) and UA, and XOR activity in kidney were measured as previously described.11
Immunofluorescence studyImmunofluorescence studies were performed as described previously.20 To evaluate the stainings of nephrin, podocin and podoplanin, the staining was graded semi-quantitatively mainly as described previously21 with some revisions: score 4, normal continuous staining; score 3, the area in which the staining was altered covered<25% of the glomerular tuft area; score 2, 25–50%; score 1, 50–75%; score 0, 75–100%. To evaluate the staining of desmin, score 0, no staining in the glomerulus; score 1, staining covered<25% of the glomerular tuft area; score 2, 25–50%; score 3, 50–75%; score 4, 75–100%. A score was assigned to an individual glomerulus. Twenty glomeruli of each rat were scored, and the mean value was considered as the score for the individual. The score for each group is shown as mean±SD. Antibodies used in this study are as follows: mouse anti-nephrin monoclonal antibody,20,22 rabbit anti-nephrin antibody,20 rabbit anti-podocin antibody18 and rabbit anti-podoplanin antibody,23 which were prepared in our laboratory, and rabbit anti-desmin antibody, which was purchased from Chemicon International Inc.
Real-time PCRQuantitative real-time PCR analyses with RNA samples of mouse kidney and human cultured podocytes were performed as described previously.18 Fold change in the expression of target genes compared with GAPDH was calculated and expressed as mean±SD for each group. The primers used in this study are as follows (Accession No., forward, reverse): GAPDH, AY618199, CTCCACTCACGGCAAATTCAA, GGATGACCTTGCCCACAGC; GAPDH, AB062273, TTCCACCCATGGCAAATTCCA, GGATGACCTTGCCCACAGC; Desmin, AK131922, CAGTCCTACACCTGCGAGAT, GGCTGGTTTCTCGGAAGTTG; Nephrin, AF190637, CTGACTGGGCTGAAGCCTTCT, AAGAGCACAGGCAGAGGGG; Podoplanin, NM006474, CCAGCGAAGACCGCTATAAG, ATGATTCCAACCAGGGTCAC; Synaptopodin, NM007286, GCCTGCCACTTTCTAGCATC, CAGGGAGCTGGACATGAAAT.
Statistical analysisStatistical significance was evaluated using the unpaired t test or Mann–Whitney U test. Values were expressed as mean±SD. Data were analysed with GraphPad Prism 5.0 software (San Diego, CA, USA). P-value<0.05 was regarded as statistically significant.
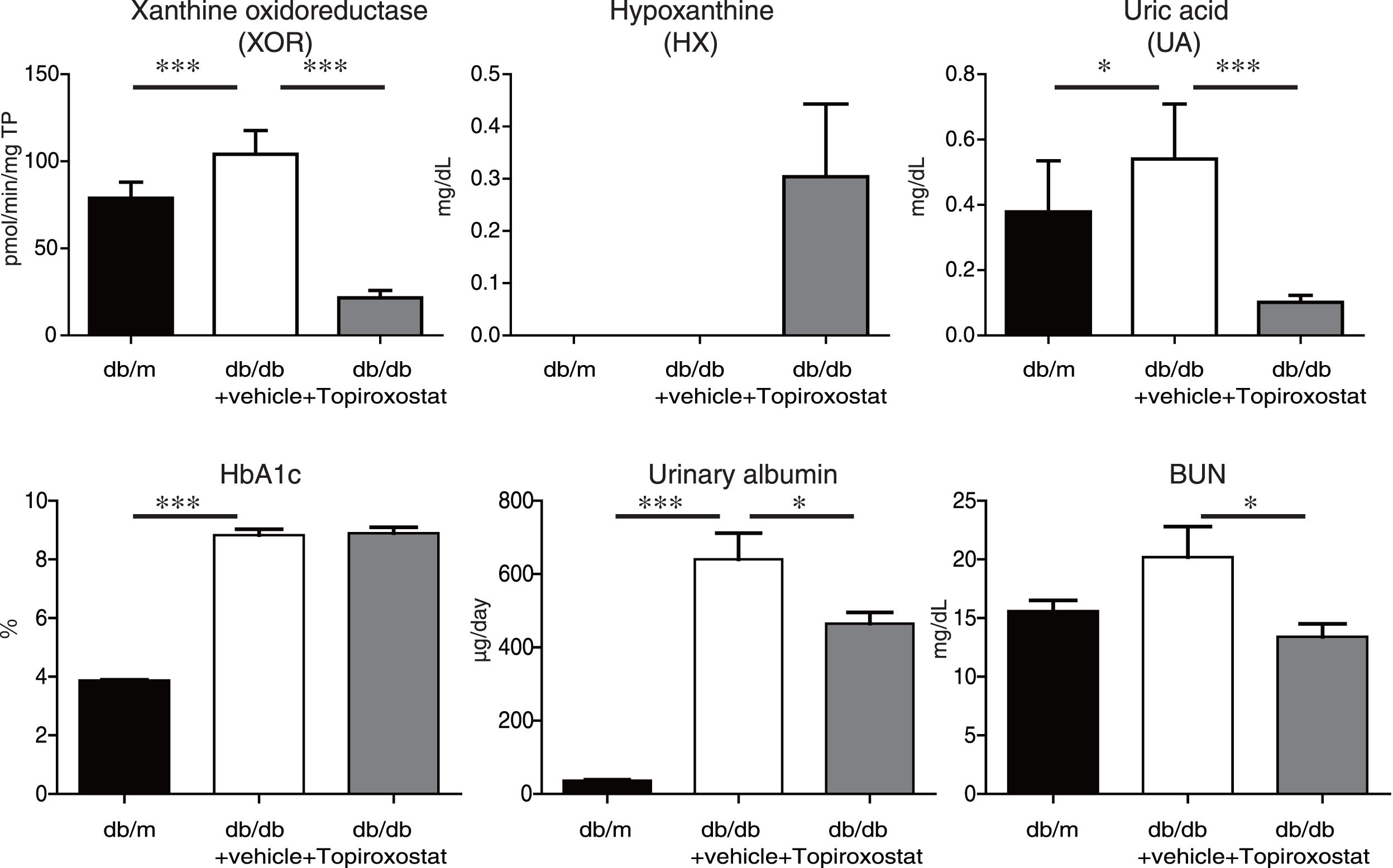
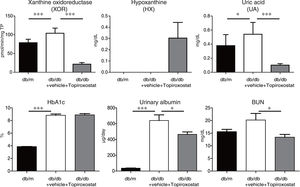
ResultsTopiroxostat treatment ameliorated albuminuria in db/db micePlasma XOR activity, and the plasma levels of hypoxanthine and UA in db/db mice were analysed at the age of 13 weeks of db/db mice. Plasma XOR activity and plasma UA level were increased, and topiroxostat treatment suppressed the plasma XOR activity and reduced plasma UA level in db/db mice. Topiroxostat treatment increased the serum level of HX (Fig. 1, upper panel). Serum Hb1Ac level and the amount of urinary albumin excretion were clearly increased at the age of 13 weeks of db/db mice. Although topiroxostat treatment did not affect HbA1c level, the treatment reduced the amount of urinary albumin excretion (Fig. 1, lower panel). These observations suggested that topiroxostat has a protective role to kidney.
The effect of topiroxostat on albuminuria in db/db mice. Plasma XOR activity and plasma UA level were increased in db/db mice, and topiroxostat treatment suppressed the plasma XOR activity and reduced plasma UA level in db/db mice. Topiroxostat treatment promoted the serum level of HX (upper panel). Serum Hb1Ac level and the amount of urinary albumin excretion were clearly increased in db/db mice. Topiroxostat treatment did not affect HbA1c level, while the treatment reduced the amount of urinary albumin excretion in db/db mice (lower panel). Data are shown as mean±SD. *P<0.05, **P<0.01, ***P<0.001.
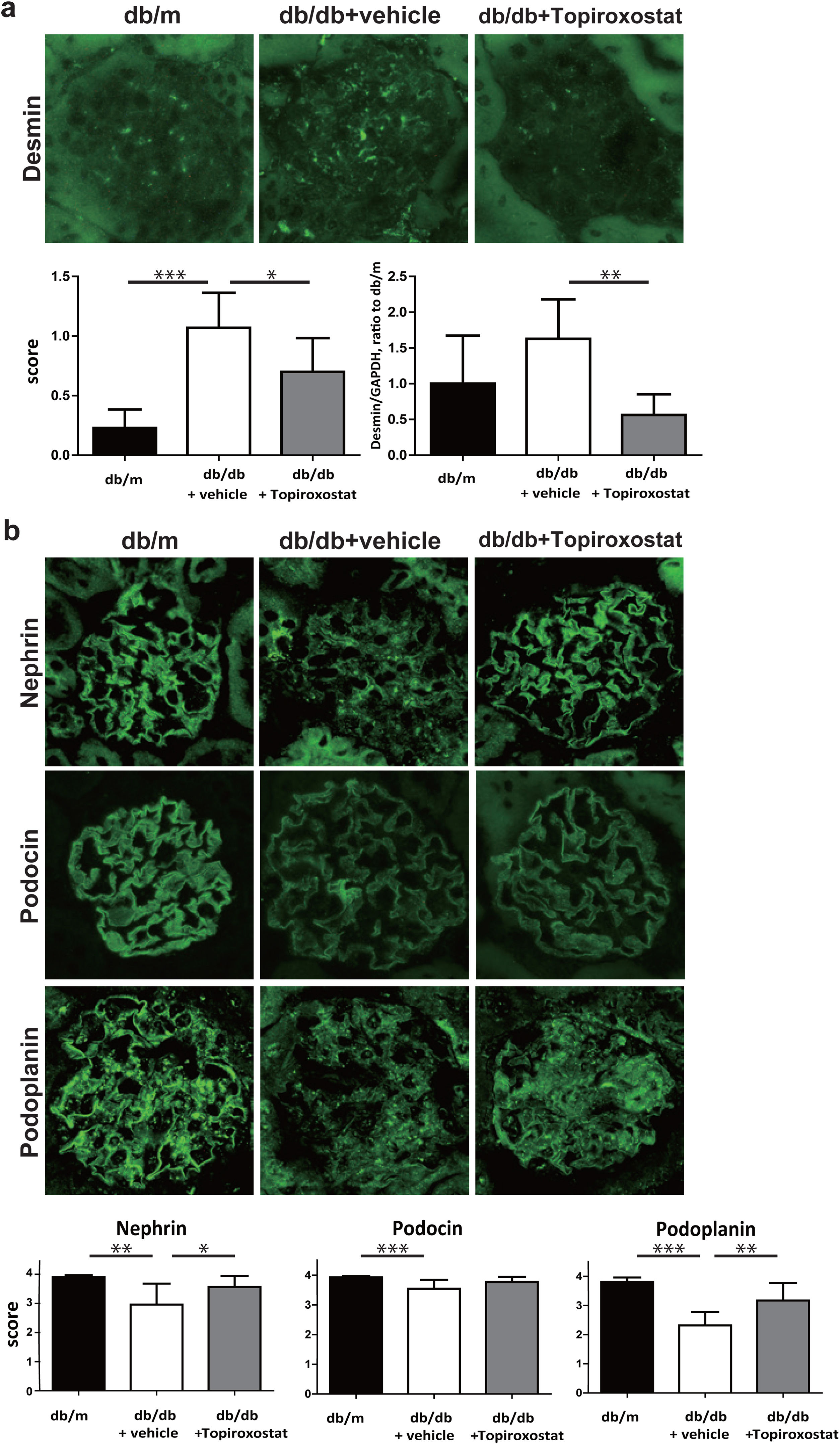
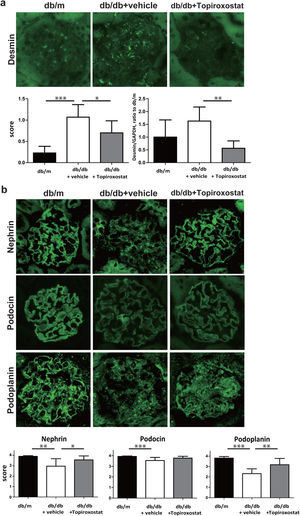
Next, the effect of topiroxostat on podocyte of db/db mice was investigated. The expression of desmin, a marker of podocyte injury was increased in db/db mice. Topiroxostat treatment reduced the immunostaining and the mRNA expression of desmin (Fig. 2a). The podocyte functional molecules, nephrin, podocin and podoplanin were detected as a continuous linear pattern along capillary loop in db/m mice. The stainings of these molecules were observed as a discontinuous dot-like pattern in db/db mice, and the staining intensity of these molecules was clearly low. Topiroxostat treatment restored the expression of nephrin (P<0.05) and podoplanin (P<0.01) (Fig. 2b).
The effect of topiroxostat on the expression of desmin and podocyte functional molecules in db/db mice. (a) Representative IF finding of desmin (upper panel), and the semi-quantitative data of immunostaining and the data of real-time PCR (lower panel). The immunostaining of desmin was increased in db/db mice (db/db+vehicle). Topiroxostat treatment reduced the expressions of desmin (db/db+Topiroxostat). (b) Representative IF findings of podocyte functional molecules (upper panel), and the semi-quantitative data (lower panel). Nephrin, podocin and podoplanin were observed as a continuous linear pattern along capillary loop in db/m. The stainings of these molecules were detected as discontinuous dot-like pattern in db/db mice, and the staining intensity of these molecules was clearly low. Topiroxostat treatment restored the expression of nephrin and podoplanin. Data are shown as mean±SD. (*P<0.05, **P<0.01, ***P<0.001).
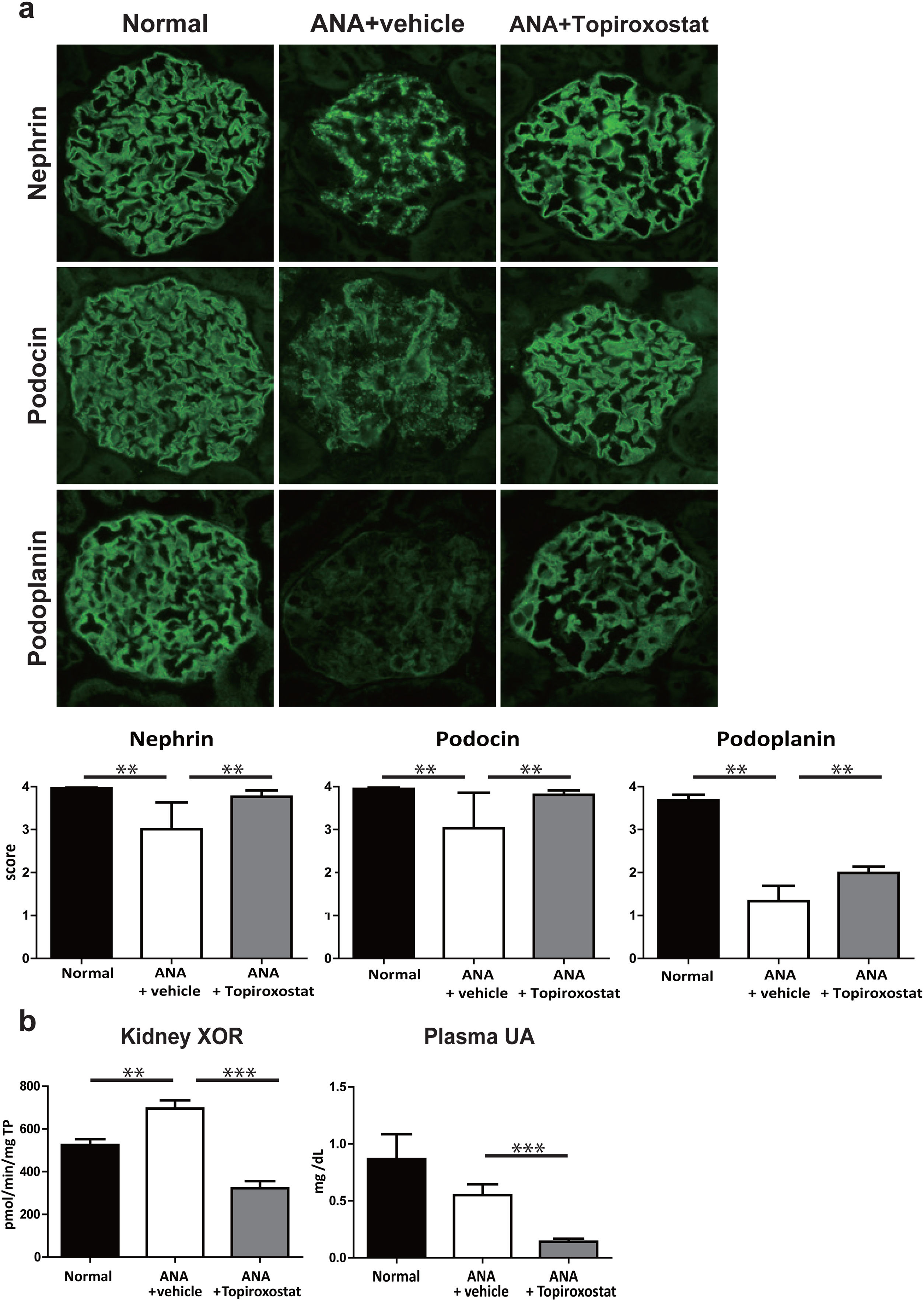
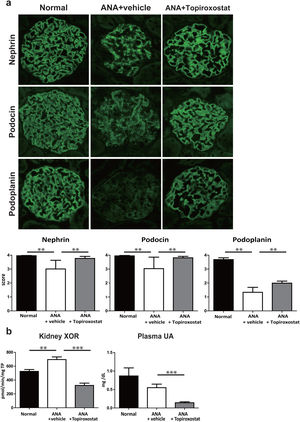
Next, to further investigate the effect of topiroxostat on podocyte, we analysed the expression of podocyte functional molecules in rats with podocyte injury caused by anti-nephrin antibody injection. The staining patterns of nephrin, podocin and podoplanin shifted to a dot-like discontinuous pattern and the staining intensity of these molecules were clearly decreased on day 5 after anti-nephrin antibody injection. Topiroxostat treatment restored the expressions of all of these molecules (P<0.01) (Fig. 3a). XOR activity in kidney was increased in this podocyte injury model. Topiroxostat treatment reduced the XOR activity in kidney and plasma UA level (Fig. 3b).
The effect of topiroxostat in anti-nephrin antibody (ANA)-induced podocyte injury. (a) Representative IF findings (upper panel), and the semi-quantitative data of immunostaining (lower panel) of podocyte functional molecules. Nephrin, podocin and podoplanin were observed as a continuous linear pattern along capillary loop in normal rat (Normal). The stainings shifted to a dot-like discontinuous pattern, and the intensity was reduced in the rats with ANA-induced podocyte injury (ANA+vehicle). Topiroxostat treatment restored the expression of all of these molecules, nephrin, podocin and podoplanin (ANA+Topiroxostat). (b) XOR activity and plasma UA level. XOR activity in kidney was increased. Topiroxostat treatment reduced the XOR activity in kidney and plasma UA. Data are shown as mean±SD. (**P<0.01, ***P<0.001).
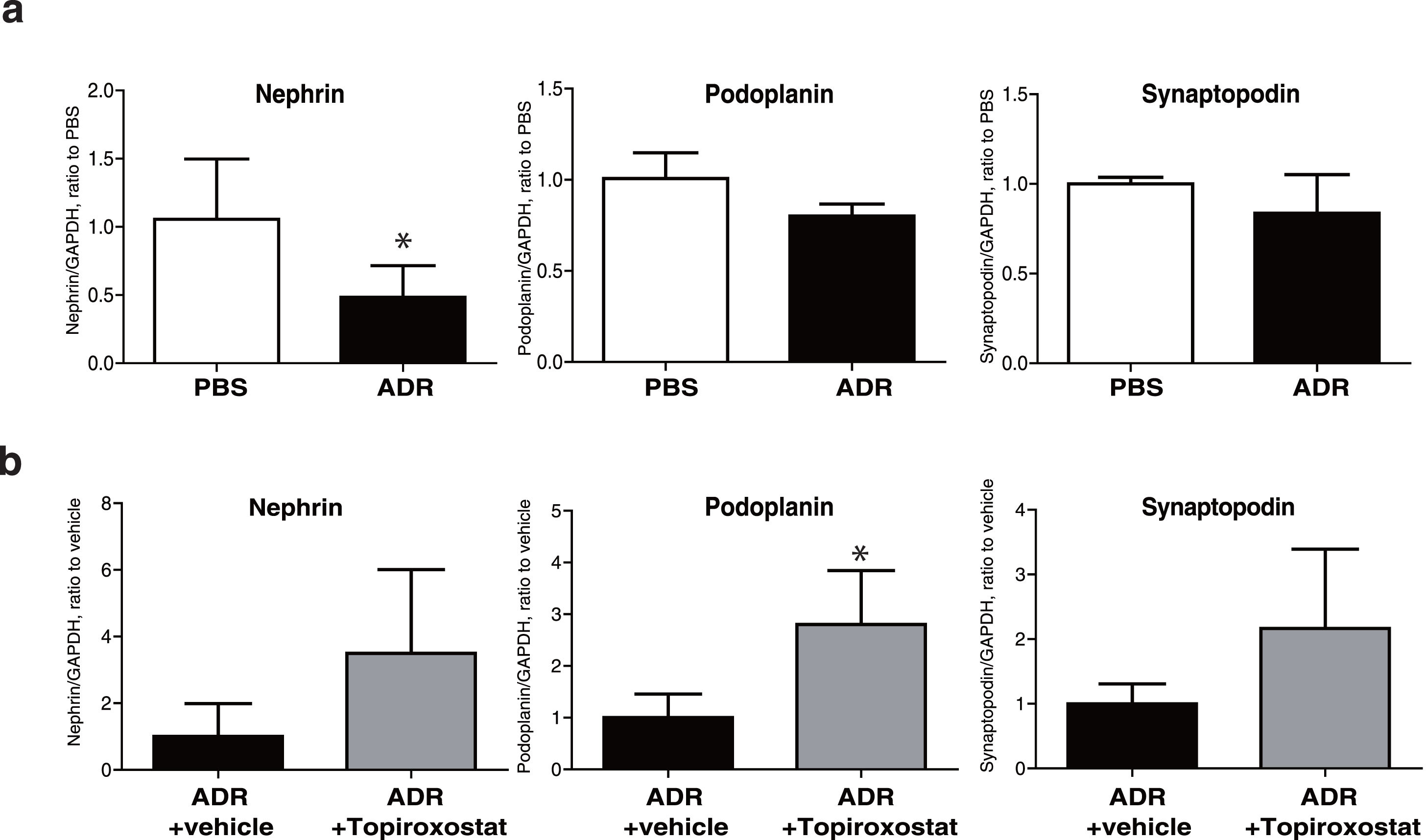
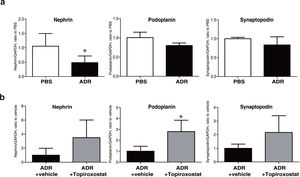
To investigate the direct effect of topiroxostat on podocyte, we analysed mRNA expressions of podocyte functional molecules, and we found that mRNA expression of nephrin was decreased in podocyte treated with adriamycin (Fig. 4). Topiroxostat treatment restored mRNA expression of podoplanin (Fig. 4b).
mRNA expression of podocyte functional molecules in human cultured podocytes. (a) mRNA expression of nephrin, podoplanin, and synaptopodin in cultured podocytes treated with adriamycin (ADR). The mRNA expression of nephrin was decreased. (b) The effects of topiroxostat on the mRNA expression of nephrin, podoplanin and synaptopodin. Topiroxostat treatment increased the mRNA expression of podoplanin in podocytes treated with ADR. Data are shown as mean±SD (n=3). (*P<0.05).
Some of recent studies showed that topiroxostat, a XOR inhibitor, ameliorated proteinuria of several types of kidney diseases.7–13 However, its pharmacological mechanism on proteinuria is not fully understood yet. In this study, first we showed that topiroxostat treatment ameliorated albuminuria in db/db mice, a diabetic nephropathy model (Fig. 1). The results coincide with the previous report.11 Although the pathogenic mechanism of proteinuria is still controversial, it is understood that proteinuria in several types of glomerular diseases results from podocyte injury. To verify podocyte injury in db/db mice, we analysed the expression of desmin, a marker of podocyte injury16 in db/db mice at the age of 13 weeks, when severe proteinuria was detected. We observed that immunostaining of desmin were increased (Fig. 2a), which indicating that podocyte injury is evident in db/db mice. Then, we analysed the effect of the topiroxostat on the podocyte injury. Since we have previously reported that the topiroxostat treatment for 4 weeks ameliorated hypertrophy of the glomeruli in the diabetic mice,12 we analysed the effect of the topiroxostat treatment for 4 weeks also in this study, and showed that the treatment suppressed the expression of desmin.
Podocyte is characterized by its unique interdigitating foot processes covering outer side of glomerular capillary wall. It is understood that a slit diaphragm connecting neighbouring foot processes functions as a final barrier to prevent proteinuria.14 Nephrin is a transmembrane protein constituting the extracellular site of slit diaphragm, and is understood to be a key molecule for maintaining the barrier function of slit diaphragm. Podocin is a scaffolding protein interacting with nephrin.14 Podoplanin is a transmembrane protein identified as a critical molecule for maintaining the interdigitation of foot processes.24 In this study we analysed the expressions of nephrin, podocin and podoplanin in nephrotic stage of db/db mice. We demonstrated that immunostaining of these molecules are clearly altered, which suggesting that the alterations of the foot process structure and dysfunction of slit diaphragm participate in the development of proteinuria in db/db mice. Then, we showed that topiroxostat treatment restored the reduction of nephrin and podoplanin in db/db mice, although the effect of topiroxostat on the podocin expression was not statistically proven (Fig. 2b). It is conceivable that topiroxostat ameliorated albuminuria of db/db mice by its protective effect on these functional molecules of podocyte.
Next, to further analyse the effect of topiroxostat on podocyte, the podocyte injury model caused by the injection of anti-nephrin antibody was adopted. We have previously reported that nephrin and other slit diaphragm-associated molecules are downregulated, and the localizations of these molecules are clearly altered in this model. The podocyte injury was induced by the stimulation to a key molecule of slit diaphragm, without complements or any inflammatory factors. Although the pathogenic mechanism of this podocyte injury is not fully clarified yet, it was reported that some of signalling cascades participate in the development of the podocyte injury.25,26 Since the alterations of the podocyte functional molecules in this model are more evident than in db/db mice, it is assumed that this model is more advantageous to analyse the effect of topiroxostat on podocyte. We observed that nephrin, podocin and podoplanin were expressed along capillary loop in normal rat glomeruli, and their stainings shifted to discontinuous dot-like pattern and their staining intensities were clearly decreased on day 5 in rats injected with the anti-nephrin antibody. We showed here that topiroxostat treatment attenuated the alterations of all of these functional molecules including podocin (Fig. 3a). It is conceivable that topiroxostat has an effect protecting slit diaphragm.
To elucidate the pharmacological mechanism of topiroxostat, we analysed the XOR activity in kidney in the anti-nephrin antibody-induced podocyte injury model. We observed that the XOR activity in kidney is evidently increased in this podocyte injury model, and the topiroxostat treatment reduced the activity in kidney (Fig. 3b). Xanthine oxidase (XO), a subtype of XOR is known to produce superoxide. Our group previously reported that topiroxostat treatment reduced the staining of nitrotyrosine, a marker of oxidative stress in glomeruli.12 Kawamorita et al. reported that the increase of oxidative stress in puromycin aminonucleoside nephrosis was ameliorated by the topiroxostat treatment.10 Although the precise pharmacological mechanism of topiroxostat in the podocyte injury of this model is unclear, it is assumed that increase in XO activity and the consequent increase of superoxide at local area of kidney participate in the development of the podocyte injury, and that topiroxostat attenuates the podocyte injury by lowering the production of superoxide. The results suggested that increase of superoxide in podocyte is involved in the development of the podocyte injury induced by the stimulation to the slit diaphragm.
In this study, finally to verify whether topiroxostat directly affects podocyte, we analysed the effect of topiroxostat on the expressions of nephrin, podoplanin and synaptopodin, one of critical actin-associated molecules,27 in cultured podocytes. To investigate the effect on human cell, a cell line of immortalized human cultured podocyte was used. The topiroxostat treatment promoted the expression of podoplanin in podocyte treated with adriamycin, a reagent used to induce FSGS like podocyte injury in vivo21 (Fig. 4). It was also observed that the topiroxostat treatment increased the expression of nephrin and synaptopodin, although the increases of these molecules were not statistically proven. These observations indicated that topiroxostat directly affects podocyte, and that topiroxostat has an effect to stabilize podoplanin.
The effect of topiroxostat on podoplanin was clearly demonstrated in all experiments in this study. Podoplanin was originally identified as a critical molecule in maintaining the specialized foot process structure of podocyte, and recently is widely known as a lymphatic endothelial cell marker.28 Matsui et al reported that the stimulation to podoplanin led to proteinuria and effacement of foot processes in rats.24 We have reported that podoplanin is widely expressed along whole cell surface of podocyte and plays a role in maintaining cytoskeletal structure of podocyte by interacting with ezrin.29 It is estimated that the alteration of podoplanin is an important initiation event in several types of podocyte injury.23,29,30 It is conceivable that topiroxostat has an effect attenuating the initiation event of podocyte injury.
ConclusionIn conclusion, the present study showed that topiroxostat has a direct effect on podocyte and ameliorated proteinuria by inhibiting the reduction of nephrin and podocin, key molecules of slit diaphragm, and podoplanin, a critical molecule maintaining foot process structure.
Ethics approvalThis article does not contain any studies with human materials.
Conflict of interestThe authors have no conflict of interest of disclose.
The authors wish to thank Ms. Yukina Kitazawa for her excellent technical assistance.