Peritoneal dialysis is an important form of kidney replacement therapy. Most patients presenting with an unplanned, urgent need for dialysis are prescribed haemodialysis, leading to peritoneal dialysis underutilisation. Urgent-start peritoneal dialysis refers to treatment that is commenced within 2 weeks of catheter placement. Urgent-start peritoneal dialysis represents an efficacious, cost-effective alternative to the conventional approach of commencing dialysis. There is a paucity of evidence to guide management, however experience with the technique is increasing. This article overviews the rationale and practical application of urgent-start peritoneal dialysis.
La diálisis peritoneal es una forma importante de terapia de reemplazo renal. A la mayoría de los pacientes que presentan una necesidad urgente e imprevista de diálisis se les prescribe hemodiálisis, lo que lleva a la infrautilización de la diálisis peritoneal. La diálisis peritoneal de inicio urgente se refiere al tratamiento que se inicia dentro de las 2 semanas posteriores a la colocación del catéter. La diálisis peritoneal de inicio urgente representa una alternativa eficaz y rentable al enfoque convencional de iniciar la diálisis. Hay escasez de evidencia para guiar el manejo, sin embargo, la experiencia con la técnica está aumentando. Este artículo describe la justificación y la aplicación práctica de la diálisis peritoneal de inicio urgente.
Peritoneal dialysis (PD) is an important form of kidney replacement therapy accounting for 11% of chronic dialysis patients worldwide.1 Relative PD utilisation is declining despite improved cost-effectiveness and quality of life compared with haemodialysis (HD) and similar clinical outcomes.1–3 Patients presenting with an unplanned, urgent need for dialysis are typically commenced on HD via a central venous catheter, both in the context of acute kidney injury (AKI) and end-stage kidney disease (ESKD).3–11 The infrequent use of PD in this situation hinders its wider uptake. Approximately 40% of chronic dialysis patients commence therapy in an unplanned fashion, for reasons including poor follow-up, late referral to nephrology, and acute hospitalisation.12 Only two-thirds of patients with ESKD who plan to commence PD as their preferred dialysis modality actually do so, most commonly due to an unexpected urgent need for treatment.13 Furthermore, individuals commenced on HD that subsequently need long-term dialysis rarely ever transition to PD.3,14
Unplanned, acute PD is emerging as an alternative to HD for patients requiring dialysis when established, functional dialysis access is absent. Experience with this form of PD, referred to as ‘urgent-start PD’ (USPD), has grown over the past decade.5,7,11 Proposed advantages include avoidance of temporary HD catheters, a reduced number of access procedures, convenience, lower costs, and greater ability to recruit patients onto long-term PD.11,15 The potential benefits of USPD have been particularly emphasised amid resource shortages encountered during the coronavirus disease 2019 (COVID-19) pandemic.1,16,17 USPD has traditionally been avoided for a number of reasons. International guidelines recommend delaying dialysis for 2 weeks after PD catheter insertion where practicable because of increased risk of mechanical complications.1,5,18–20 Other concerns which are frequently raised in practice include lack of experience and familiarity, perceived superiority of HD in acute settings, and logistical difficulties.
USPD has not been widely studied and its performance compared with conventional approaches such as urgent HD and delayed, planned PD remains uncertain.21 Published practice patterns are highly variable. This article provides a comprehensive, contemporary review of the rationale and practical application of USPD.
DefinitionUSPD is defined as commencement of PD within 2 weeks following PD catheter placement.1,3 As a therapy, USPD may be broadly applicable for both patients with AKI and ESKD. USPD is sometimes referred to as ‘acute-start PD’, ‘acute PD’ or ‘early-start PD’.7,16,21,22
Although the 2-week criteria is most widely accepted, various alternative time-based definitions can be found in the literature.3 Some groups contend that USPD not apply to instances where bridging HD is utilised, or where emergency dialysis is required within 2 days of catheter placement, in which cases HD may be more appropriate.3,23 ‘Emergency-start PD’ is sometimes used to characterise PD that is undertaken emergently within hours.
Indications and contraindicationsPatients who are candidates for PD and who have the standard indications for dialysis may be appropriate for USPD. USPD is a feasible strategy for patients with ESKD, however it should be reserved for those anticipated to continue on PD longer-term. USPD is also recommended by the International Society of Peritoneal Dialysis (ISPD) and Caring for Australians and New Zealanders with Renal Impairment (CARI) guidelines as an acceptable renal replacement therapy for patients with AKI.19,24 This recommendation is resource-dependent because its use in AKI is poorly understood and the median time to catheter insertion in centres reporting on USPD programmes is between 2 and 6 days; many patients are unable to wait this long for dialysis.3,7,11,25 USPD remains a preferred management approach for children with AKI and in many developing countries.33,34
There are few absolute contraindications to USPD, and relative contraindications are similar to those for conventional PD (Table 1). Careful patient selection is crucial. USPD is often avoided in patients with life-threatening indications for emergency dialysis, such as severe hyperkalaemia or pulmonary oedema (Table 1).1,7,25 HD is generally regarded as a safer choice in such cases due to its ability to achieve more rapid and accurate solute clearance and ultrafiltration.3,7,25,26 Prospective studies have found that several days of PD are required to reliably gain control of urea, creatinine, potassium and bicarbonate.27,28 USPD is also usually avoided in patients who are critically unwell or who have significant respiratory failure, although this is not evidence-based.1,9,14,29,30
Contraindications for urgent-start peritoneal dialysis.
| Absolute contraindications | |
| Patient not consenting | |
| Recent surgery involving large anterior abdominal incisions | There is a general consensus that operations using large abdominal wall incisions should probably invoke a minimum 2-week hiatus from PD due to the risk of fluid leak and hernia.1 |
| Relative contraindications | |
| Severe hyperkalaemia | Emergency haemodialysis is often more appropriate in such indications, as urgent-start peritoneal dialysis may be unable to safely achieve the requisite accuracy and speed of treatment. If peritoneal dialysis is chosen, regular patient reassessment is prudent. |
| Severe pulmonary oedema | |
| Severe uraemic pericarditis or encephalopathy | |
| Critical illness | Concerns about inadequacy, cachexia and protein losses. |
| Respiratory failure | Upward diaphragmatic pressure affecting lung mechanics. |
| Active intra-abdominal infection | Theoretical but unproven risk of exacerbation of infection. |
| Overlying soft tissue infection | Tenckhoff catheter should be cited in another location. |
| Uncorrected bleeding diathesis | Risk of haemorrhage after catheter implantation. |
| Significant past history of abdominal surgery or disease | Diverse anecdotal experience has seen PD to be safe and effective in the post-operative setting and in many patients with structural lesions such as hernias. PD is also feasible in patients with a distant history of major abdominal surgery, though peritoneal membrane function is difficult to predict.1 Recent cardiothoracic surgery, neurosurgery and laparoscopic or minor open abdominal surgery do not preclude USPD.1 In patients with previous abdominopelvic surgery, surgical PD catheter placement allowing direct vision is advisable as opposed to percutaneous insertion. |
| Large abdominal wall hernia | |
| Recent abdominal surgery | |
| Perioperative management | |
Patients who are seriously ill or who are commenced on PD for life-threatening indications should be regularly reassessed and quickly changed to HD if clinical improvement is inadequate.1 They may be transitioned back to PD once medically stabilised and it is safe to do so.1,31,32
Preparation and catheter placementThe success of USPD depends on functioning access to the peritoneal cavity which is obtained in the form of a PD catheter.
PreparationPatient preparation is often limited due to the need to act urgently. Where time permits, pre-procedure preparation should include mapping an exit site location and attempts to minimise constipation. Bowel preparation regimens vary by institution but stools should be liquid at the time of catheter insertion if possible. The bladder should be empty to reduce the risk of bladder injury.
Depending on the technique chosen, PD catheter insertion may occur at the bedside, in a fluoroscopy suite or operating theatre; a clean workspace and sterile precautions are mandatory. Peri-procedural intravenous antibiotic prophylaxis is recommended.21
Patients with ESKD who are known to the nephrology service and are electively commencing therapy may be suitable for outpatient USPD if appropriate arrangements are in place.22
Catheter insertion techniquePD catheters are broadly separated into rigid non-cuffed catheters or flexible cuffed catheters. Rigid non-cuffed catheters can be expeditiously placed into the peritoneal cavity at the bedside and may provide lifesaving dialysis access, however their use is declining and is largely restricted to resource-limited settings. These straight, non-tunnelled catheters are temporary and can only be left in place for a few days due a high incidence of peritonitis. Other options in exceptional circumstances include improvised nasogastric tubes and surgical drains.29
Tunnelled Tenckhoff PD catheters are the preferred choice for USPD when available.21,29 They are silicone-plastic cuffed catheters which are increasingly available and, compared to older rigid catheters, associated with fewer complications and better function.14 Various Tenckhoff catheter designs are used however none has been conclusively shown to be superior to the others1; double-cuffed catheters are generally preferred based on outcomes in chronic PD patients.1,20
Tenckhoff catheters may be implanted using both percutaneous and surgical methods. There appears to be no significant difference in clinical outcomes between insertion techniques.3,21,29,35–37 However, percutaneous catheter placement has been associated with improved cost-effectiveness and logistical efficiency.18,31,37 The most important factors dictating success are probably local experience and resources.3,21,29,36 Outcomes are comparable across operators, including interventional nephrologists, radiologists and surgeons.21,29,37
Initial dialysis prescriptionRationalePrescribing dialysis depends on a number of factors, including a patient's residual renal function, body size, the clinical context and the desired effects of treatment. USPD requires an adequate dialysis prescription balanced against the risk of treatment-related complications. The fundamental reservation is the need to reduce the chance of peri-catheter dialysate leak by minimising intraabdominal pressure while the catheter tunnel is allowed to heal. Conversely, this is a lesser concern in patients with chronic kidney disease (CKD) who are able to commence non-urgent, incremental, planned PD several weeks after a PD catheter has been implanted. Solute clearance and net ultrafiltration are dependent on the peritoneal concentration gradient which can be regulated by adjusting PD dwell volumes, the number and duration of exchanges, and solution tonicity.
Patients with AKI are conceptually quite different to those commencing dialysis for ESKD. They require USPD with greater precision, higher clearance and faster action than is typically necessary for patients who are systemically well with gradually deteriorating renal function. Peritoneal membrane transport function is unknown at the outset of USPD, since a peritoneal equilibration test is properly performed after at least 1 month of therapy, so clinicians must exercise their judgement on a case-by-case basis when prescribing treatment.
Prescribing USPD in patients with end-stage kidney diseaseA range of USPD prescriptions have been published in the literature for patients with ESKD.3,4,18,35,38–40 Two generic methods are usually tried, including continuous ambulatory PD (CAPD) and automated PD (APD), employing a variety of exchange volumes and frequencies. CAPD refers to PD exchanges administered manually by patients or staff, while APD refers to PD dwells that are delivered by an automated machine cycler. Both techniques are accepted by guidelines and yield similar outcomes when adapted for USPD.1,21 APD is more commonly employed in this situation and is preferred where resources allow because it is more convenient and is associated with a lower rate of early peritonitis.9,14,21,40 Both modalities should be prescribed using frequent, small-volume exchanges with patients maintained in the supine position whenever dialysate is instilled in the peritoneal cavity, as this reduces intra-abdominal pressure and theoretically the chance of leak.1,4,22
Determination of adequate USPD should preferably be based on clinical features, since clinical status is a patient-oriented indicator of effective dialysis.24,41 Formal biochemical markers of dialysis adequacy may be supplemented as a helpful guide. Based on randomised trial data in the chronic CAPD and APD populations, international guidelines recommend a minimum weekly Kt/V of 1.7 where feasible.22,42–49Kt/V is a dimensionless ratio representing urea clearance relative to urea distribution volume: the actual value is not routinely measured in practice during USPD because of time constraints and inaccuracy (Table 2).9,14 A rough short-term estimation can be anticipated for most patients given that Kt/V is a product of total drain volume and urea concentration, and that clearance is reasonably predictable with short dwell times. Minimally adequate dialysis in an average person with anuria equates to at least 1000mL of ultrafiltration daily and requires roughly 10L of dialysate per day.25,41,50–54
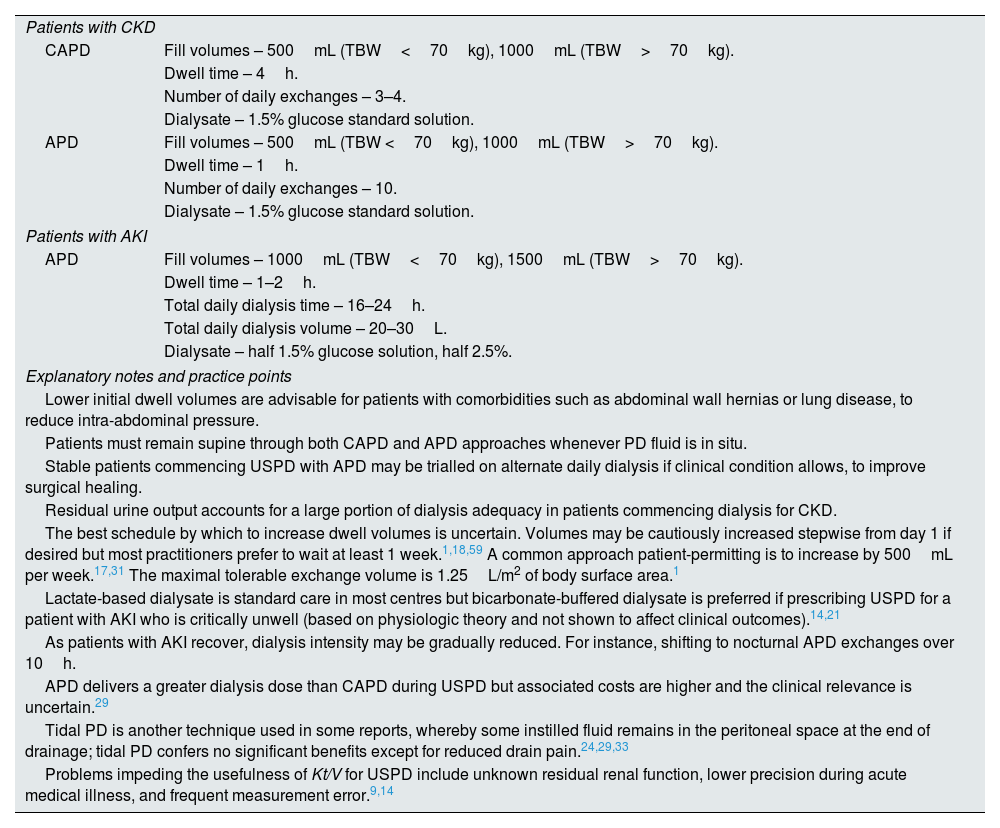
Suggested initial urgent-start peritoneal dialysis prescriptions.
| Patients with CKD | |
| CAPD | Fill volumes – 500mL (TBW<70kg), 1000mL (TBW>70kg). |
| Dwell time – 4h. | |
| Number of daily exchanges – 3–4. | |
| Dialysate – 1.5% glucose standard solution. | |
| APD | Fill volumes – 500mL (TBW <70kg), 1000mL (TBW>70kg). |
| Dwell time – 1h. | |
| Number of daily exchanges – 10. | |
| Dialysate – 1.5% glucose standard solution. | |
| Patients with AKI | |
| APD | Fill volumes – 1000mL (TBW<70kg), 1500mL (TBW>70kg). |
| Dwell time – 1–2h. | |
| Total daily dialysis time – 16–24h. | |
| Total daily dialysis volume – 20–30L. | |
| Dialysate – half 1.5% glucose solution, half 2.5%. | |
| Explanatory notes and practice points | |
| Lower initial dwell volumes are advisable for patients with comorbidities such as abdominal wall hernias or lung disease, to reduce intra-abdominal pressure. | |
| Patients must remain supine through both CAPD and APD approaches whenever PD fluid is in situ. | |
| Stable patients commencing USPD with APD may be trialled on alternate daily dialysis if clinical condition allows, to improve surgical healing. | |
| Residual urine output accounts for a large portion of dialysis adequacy in patients commencing dialysis for CKD. | |
| The best schedule by which to increase dwell volumes is uncertain. Volumes may be cautiously increased stepwise from day 1 if desired but most practitioners prefer to wait at least 1 week.1,18,59 A common approach patient-permitting is to increase by 500mL per week.17,31 The maximal tolerable exchange volume is 1.25L/m2 of body surface area.1 | |
| Lactate-based dialysate is standard care in most centres but bicarbonate-buffered dialysate is preferred if prescribing USPD for a patient with AKI who is critically unwell (based on physiologic theory and not shown to affect clinical outcomes).14,21 | |
| As patients with AKI recover, dialysis intensity may be gradually reduced. For instance, shifting to nocturnal APD exchanges over 10h. | |
| APD delivers a greater dialysis dose than CAPD during USPD but associated costs are higher and the clinical relevance is uncertain.29 | |
| Tidal PD is another technique used in some reports, whereby some instilled fluid remains in the peritoneal space at the end of drainage; tidal PD confers no significant benefits except for reduced drain pain.24,29,33 | |
| Problems impeding the usefulness of Kt/V for USPD include unknown residual renal function, lower precision during acute medical illness, and frequent measurement error.9,14 | |
CKD – chronic kidney disease, AKI – acute kidney injury, PD – peritoneal dialysis, APD – automated peritoneal dialysis, CAPD – continuous ambulatory peritoneal dialysis, USPD – urgent-start peritoneal dialysis, and TBW – total body weight.
Because patients starting dialysis for ESKD will have some residual renal function, intensive treatment is not usually required. A typical incremental urgent-start CAPD prescription begins with 3–4 low-volume daytime exchanges (Table 2). Alternatively, APD could be performed for 8–10h overnight using frequent, small exchanges. Dwell volumes should be commensurate with patient body size, although some clinicians begin with 1L exchanges for all patients.17,31 The initial PD prescription comprises low-tonicity dialysate solutions. Ongoing refinement of the USPD prescription must be individualised; a practical summary is provided in Table 3.
Prescribing USPD in patients with AKIThe optimal use of USPD in patients with AKI is contentious and physician practices and preferences vary markedly.4,9,21,55,56 Its purpose in the management of AKI is to achieve rapid stabilisation of electrolytes, acid-base balance and fluid status. Patients are often anuric and more complex than those with relatively stable ESKD, necessitating more aggressive dialysis which entails appreciably higher dwell volumes and frequency. APD is the commonest mode prescribed for AKI and should be the default over CAPD where attainable, as manual exchanges are impractical for provision of high-volume dialysis.29,33
Achieving adequate dialysis in severe AKI typically demands at least 20L of APD administered over 16–24h per day.29,33 Guidelines recommended a minimum weekly Kt/V of 2.1 based on prospective trial data, which should approximately match that dialysis regimen, but this must be considered in conjunction with clinical progress.21,29,50,57,58 Ultrafiltration is unpredictable so a baseline prescription should include an evenly divided combination of standard 1.5% and 2.5% glucose dialysate, increasing the proportion of 2.5% glucose solution as needed.17 There is inevitably some degree of trial by error; detailed notes outlining the USPD prescription for AKI are provided in Table 2. In real-world practice many ward patients will struggle to remain supine and adhere to more than 12h of continuous APD per day.17
The PD prescription should be evaluated and charted on a daily basis because patient condition changes rapidly and regular reassessment is prudent. Where solute removal or ultrafiltration is inadequate, adjustments to the PD regimen should be made (Table 3). As the patient medically stabilises the intensity of dialysis can be gradually alleviated. Frequent monitoring of serum biochemistry with electrolyte replacement should be undertaken as apropos, and concomitant loop diuretics may be used for persistently fluid-overloaded patients. If clinical improvement is unsatisfactory a transition to acute HD may ultimately be required, particularly where patients are anuric or the indication for therapy is life-threatening.
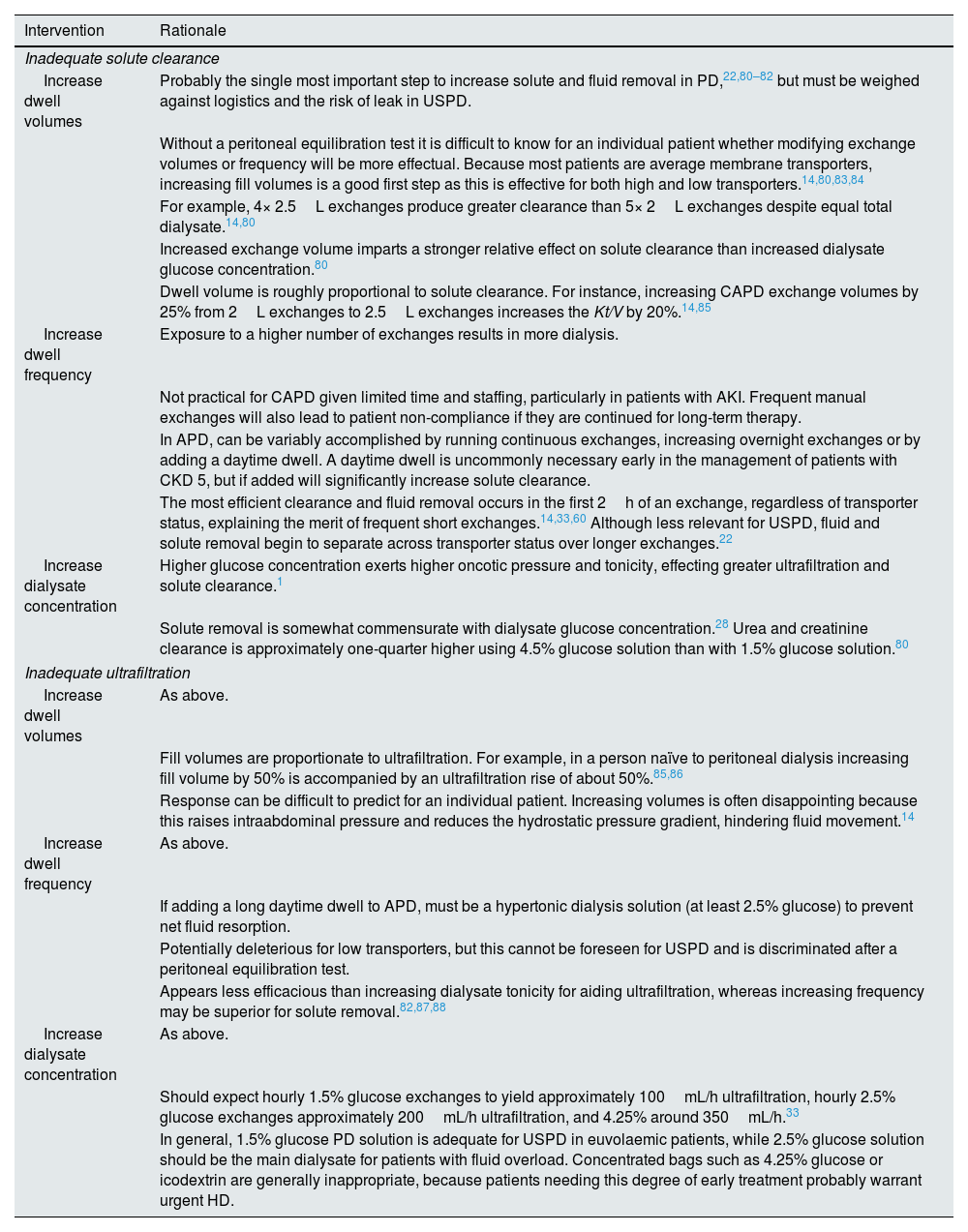
Titration of the urgent-start peritoneal dialysis prescription.
| Intervention | Rationale |
|---|---|
| Inadequate solute clearance | |
| Increase dwell volumes | Probably the single most important step to increase solute and fluid removal in PD,22,80–82 but must be weighed against logistics and the risk of leak in USPD. |
| Without a peritoneal equilibration test it is difficult to know for an individual patient whether modifying exchange volumes or frequency will be more effectual. Because most patients are average membrane transporters, increasing fill volumes is a good first step as this is effective for both high and low transporters.14,80,83,84 | |
| For example, 4× 2.5L exchanges produce greater clearance than 5× 2L exchanges despite equal total dialysate.14,80 | |
| Increased exchange volume imparts a stronger relative effect on solute clearance than increased dialysate glucose concentration.80 | |
| Dwell volume is roughly proportional to solute clearance. For instance, increasing CAPD exchange volumes by 25% from 2L exchanges to 2.5L exchanges increases the Kt/V by 20%.14,85 | |
| Increase dwell frequency | Exposure to a higher number of exchanges results in more dialysis. |
| Not practical for CAPD given limited time and staffing, particularly in patients with AKI. Frequent manual exchanges will also lead to patient non-compliance if they are continued for long-term therapy. | |
| In APD, can be variably accomplished by running continuous exchanges, increasing overnight exchanges or by adding a daytime dwell. A daytime dwell is uncommonly necessary early in the management of patients with CKD 5, but if added will significantly increase solute clearance. | |
| The most efficient clearance and fluid removal occurs in the first 2h of an exchange, regardless of transporter status, explaining the merit of frequent short exchanges.14,33,60 Although less relevant for USPD, fluid and solute removal begin to separate across transporter status over longer exchanges.22 | |
| Increase dialysate concentration | Higher glucose concentration exerts higher oncotic pressure and tonicity, effecting greater ultrafiltration and solute clearance.1 |
| Solute removal is somewhat commensurate with dialysate glucose concentration.28 Urea and creatinine clearance is approximately one-quarter higher using 4.5% glucose solution than with 1.5% glucose solution.80 | |
| Inadequate ultrafiltration | |
| Increase dwell volumes | As above. |
| Fill volumes are proportionate to ultrafiltration. For example, in a person naïve to peritoneal dialysis increasing fill volume by 50% is accompanied by an ultrafiltration rise of about 50%.85,86 | |
| Response can be difficult to predict for an individual patient. Increasing volumes is often disappointing because this raises intraabdominal pressure and reduces the hydrostatic pressure gradient, hindering fluid movement.14 | |
| Increase dwell frequency | As above. |
| If adding a long daytime dwell to APD, must be a hypertonic dialysis solution (at least 2.5% glucose) to prevent net fluid resorption. | |
| Potentially deleterious for low transporters, but this cannot be foreseen for USPD and is discriminated after a peritoneal equilibration test. | |
| Appears less efficacious than increasing dialysate tonicity for aiding ultrafiltration, whereas increasing frequency may be superior for solute removal.82,87,88 | |
| Increase dialysate concentration | As above. |
| Should expect hourly 1.5% glucose exchanges to yield approximately 100mL/h ultrafiltration, hourly 2.5% glucose exchanges approximately 200mL/h ultrafiltration, and 4.25% around 350mL/h.33 | |
| In general, 1.5% glucose PD solution is adequate for USPD in euvolaemic patients, while 2.5% glucose solution should be the main dialysate for patients with fluid overload. Concentrated bags such as 4.25% glucose or icodextrin are generally inappropriate, because patients needing this degree of early treatment probably warrant urgent HD. | |
PD – peritoneal dialysis, USPD – urgent-start peritoneal dialysis, CAPD – continuous ambulatory peritoneal dialysis, APD – automated peritoneal dialysis, CKD – chronic kidney disease, and HD – haemodialysis.
It is important to determine whether USPD is as safe and effective as conventional alternatives, such as immediate HD followed by incremental PD that is deferred until 2 weeks after catheter placement.
Early complicationsA primary deterrent to broader acceptance of USPD has been concern for increased risk of early mechanical complications. A systematic review of 15 observational studies and 1 randomised controlled trial found that, compared to a conventional strategy of waiting at least 2 weeks before PD catheter use, an urgent-start approach is associated with increased risk of peri-catheter dialysate leak (RR 3.90, 95% CI 1.56–9.78).61 In the randomised trial, 122 patients in a large tertiary centre in Australia were randomly assigned to commence PD at 1-week, 2-weeks and 4-weeks from catheter placement; the trial was stopped early due to a higher rate of complications in the 1-week group.18 Fluid leak occurred in 28.2% of the group commencing PD at 1-week, 9.5% at 2-weeks, and 2.4% at 4-weeks; differences were statistically significant. The mean time to leak onset was also significantly shorter in the 1-week group. Furthermore, 21% of patients in the 1-week group required temporary bridging with HD while only 5% converted to HD in the 2-week group.24 The risk of peri-catheter leak has not been shown in retrospective studies to be correlated with dwell volume.62–64
Compared to conventional planned PD, USPD does not appear to be associated with an increased incidence of catheter obstruction, migration, malposition, or need for catheter readjustment, nor poor effluent drainage, exit site infection, peritonitis, or bleeding.3,61,65,66 However, the risk of infection may be higher in the presence of dialysate leak.61 The incidence of early catheter flow problems is approximately 20% with both strategies.25,67
USPD versus conventional PDThe literature suggests equivalent patient-oriented outcomes between USPD and the traditional method of commencing PD more than 2 weeks after catheter placement. There is no demonstrable difference in short-term mortality, hospitalisations, or technique survival, although observational series show that patients treated with USPD receive lower mean dwell volumes and overall dialysis dose than those managed with a conventional-start approach.5,11,61,65,66,68 There is also no significant difference between USPD and conventional-start PD with respect to longer-term mortality or technique survival,5,61,62,69 though USPD may be associated with a shorter time to first rehospitalisation and a greater number of days spent in hospital at 12-months.5 Patient satisfaction has not been studied.
USPD versus urgent HDData comparing USPD with urgent HD is difficult to interpret due to considerable study heterogeneity.
There are no randomised trials comparing USPD and urgent HD in patients with ESKD, although one is currently underway.70 A Cochrane systematic review of 7 cohort studies reported equivalent technique survival, patient survival, and hospitalisation rate in both groups, but a lower incidence of bacteraemia with USPD versus acute HD.71 HD achieves superior dialysis adequacy25 and is associated with shorter hospital admission time,7 although USPD appears to be cheaper.56,58,72
A randomised trial from Vietnam reported in 2002 compared USPD to continuous renal replacement therapy (CRRT) in 70 patients with AKI and severe sepsis.34 Patients assigned to the USPD group had a mortality rate of 47% compared to 15% in the CRRT group (OR 5.1, 95% CI 1.6–16.0). Metabolic control, including clearance of creatinine and lactate and resolution of acidosis, was achieved significantly earlier in the CRRT group, and dialysis duration was shorter. The study was terminated early for safety reasons. CRRT was also more cost-effective than USPD in this trial.9,34 There are several important caveats to the study findings. Temporary rigid catheters were used as opposed to modern Tenckhoff catheters and exchanges were performed manually and were poorly documented; that said, the PD schedule was aggressive at approximately 70L of dialysate per day and the incidence of peritonitis was negligible.
A subsequent randomised trial enrolled 120 patients with AKI in Brazil, predominantly with acute tubular necrosis from sepsis or heart failure, to USPD or urgent daily intermittent HD.10 It showed no difference in mortality or infectious complications, equal control of biochemical parameters, and earlier recovery of renal function in the USPD group. Measured dialysis adequacy was lower in the USPD arm. There were methodological differences between this trial and the Vietnamese trial which potentially explain the observed outcomes for USPD. The Brazilian study excluded patients who were critically unwell or who had emergency indications for dialysis whereas the Vietnamese trial included all-comers; this subset of patients has historically received HD in most centres. The Brazilian study prescribed high-volume PD using flexible Tenckhoff catheters and automated cyclers, and patients in the intermittent HD group were assigned comparatively small treatment doses.
In patients with AKI, data from a number of other small studies suggest similar long-term mortality,5,21,38,57,73 time to recovery of renal function,38,74 haemodynamic tolerance, and cost70 between USPD and urgent HD. Ultrafiltration and dialysis adequacy are greater with HD while control of hyperkalaemia and acidosis is variable.1,21,57,75,76 It is important to recognise that many studies are confounded by selection bias and patients with life-threatening indications for dialysis were mainly excluded.77
Ongoing careIn the early phases of USPD, regular reassessment of clinical status and blood biochemistry is important, and attentive, individualised PD titration is required. Bowel habits must be monitored and aperients administered to avoid constipation. Catheter nursing care should be protocolised. Patients that develop early complications such as peri-catheter fluid leak may require cessation of PD, potentially necessitating temporary conversion to HD.63
A determination of when to discontinue PD must be made for patients with AKI while individuals commencing chronic therapy should be transitioned to an outpatient dialysis facility when appropriate, to continue nurse-led education and training and to establish a maintenance PD prescription in consultation with the treating nephrologist.
Developing an urgent-start programmeSuccessful implementation of an USPD programme requires dedicated administrative and medical support.30,78,79 The rate-limiting step is frequently the timely placement of a PD catheter, which depends on availability of proceduralists and facilities. Percutaneous insertion is possibly the favoured technique, contingent upon local expertise and resources.21 Some centres reporting on their urgent-start programmes described workflow improvements with introduction of percutaneous PD catheter insertion, usually performed by a nephrologist, by circumventing delays from obtaining surgical consultations and awaiting operating room availability.3 Other essential instruments for an USPD programme include nursing support, patient education, deliberate infrastructure, multi-specialty team collaboration and surgical backup for complex cases, and clear protocols.
ConclusionUSPD represents an opportunity to establish patients with urgent, unplanned dialysis requirements onto a safe, cost-effective, home-based therapy. It may also be undertaken as a planned strategy in some cases. USPD is an attractive approach for carefully selected patients and should be viewed as a complementary alternative to conventional strategies rather than a replacement. With a paucity of evidence to inform clinical decisions, the application of USPD depends on individual circumstances, nephrologist preferences and local practices. Collaboration with patients and caregivers is essential. On the balance of evidence the optimal candidate for USPD is perhaps the person requiring dialysis within 2 weeks but not before 2 days. Because of an increased risk of mechanical complications with USPD versus a conventional approach, patients who can delay commencing dialysis treatment for more than 2 weeks after catheter placement should probably do so, while acute HD may be more appropriate than USPD for the subgroup of patients with life-threatening AKI. Implementation of an USPD programme requires institutional support and devoted multidisciplinary cooperation. USPD can potentially alter the default approach to commencing dialysis, and its utilisation may increase as technique familiarity grows.
Conflict of interestThe authors have no relevant competing interests to declare.