Acute kidney injury (AKI) has a high prevalence in critical care patients. Early detection might prevent patients from developing chronic kidney disease and requirement for renal replacement therapy. If we compare AKI with acute coronary syndrome, in which an increase in cardiac troponin may trigger early diagnosis and therapeutic intervention, we could extrapolate a similar technique in patients with early AKI without changes in urinary frequency or serum creatinine. The objective is to identify biomarker-positive, creatinine-negative patients that would allow therapeutic interventions to be initiated before finding changes in serum creatinine, preventing kidney damage. Tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7 are cell cycle arrest biomarkers that have demonstrated, in recent clinical trials, to have good sensitivity and specificity for early detection of AKI. Other recent studies have shown that the joint use of these biomarkers with serum creatinine and urine production could improve the prognosis of AKI in critical patients. The application of these biomarkers in clinical practice would enable the early identification of patients at risk of AKI, establishing interventions that would improve the survival of renal function.
Existe una gran prevalencia del fracaso renal agudo (FRA) en pacientes críticos. La detección temprana prevendría el desarrollo de la enfermedad renal crónica y el requerimiento de terapias renales de sustitución. Si comparamos el FRA con el síndrome coronario agudo, en el que el uso de la troponina cardiaca permite el diagnóstico precoz y su consecuente terapia, se podría extrapolar técnica similar en pacientes con FRA temprano sin cambios en la frecuencia urinaria o creatinina sérica. El objetivo sería identificar a pacientes con un biomarcador positivo y la creatinina negativa que permitiera iniciar intervenciones terapéuticas antes de objetivar cambios en la creatinina sérica, previniendo el daño renal. El inhibidor tisular de metaloproteinasa-2 y la proteína de enlace 7 del factor de crecimiento insulínico, son biomarcadores de detención del ciclo celular, que han demostrado, en estudios recientes, tener adecuada sensibilidad y especificidad para la pronta identificación del FRA. Otros estudios recientes han mostrado que el uso conjunto de estos biomarcadores con la creatinina sérica y la producción de orina, pudieran mejorar el pronóstico del FRA en pacientes críticos. La aplicación de estos biomarcadores en la práctica clínica permitiría la identificación precoz de pacientes con riesgo de FRA estableciendo intervenciones que mejorarían la supervivencia de la función renal.
There is an increased incidence and prevalence of acute kidney injury (AKI) in the critical care population that influences the probability of survival a complicates comorbidities.1 Acute kidney injury implies reversible kidney damage that occurs in a particular time period. A distinction should be made between kidney injury and dysfunction. The rise in serum creatinine lags behind changes in glomerular filtration rate and depends on its own generation and steady state. This influences the rate of excretion setting the serum creatinine at a higher value. An important goal in the past few years has been to identify a biomarker sensitive enough to detect kidney injury and at the same time complement or substitute the dependency on serum creatinine and or urine output. If we compare AKI with acute coronary syndrome, in which a increase in troponin may trigger a diagnosis and therapeutic intervention, the diagnosis of AKI could be made in the absence of oliguria or increase creatinine level. The fact that AKI (subclinical) is not clinically determined does not mean that the kidney is intact with normal glomerular filtration rate. Sub clinical AKI may be identified by the isolation of new biomarkers. The goal is to identify a biomarker positive creatinine negative patient that has sustained injury to the kidney but still is in a pre-clinical phase based on serum creatinine. This new approach will identify more conditions causing AKI changing its incidence, prevalence and triggering interventions prior to changes in serum creatinine that will possibly improve renal outcomes. The end result will be to distinguish between renal function loss and AKI with tubular damage. All this will improve the time table for prompt recognition of pre-renal versus non renal causes of renal failure. A practical way to apply this concept will be to established different phases of dysfunction: Phase 1) no AKI with no biomarker detection. Phase 2) AKI with tubular damage and biomarker positive (subclinical AKI). Phase 3) AKI with filtration dysfunction (RIFLE/AKIN/KDIGO positive). Phase 4) with tubular damage, biomarker positive and filtration dysfunction (RIFLE/AKIN/KDIGO positive). These pre injury phases can also be define as acute kidney stress.2
Efforts for discovering new biomarkers for AKI have been curtailed by their sensitivity and specificity to identified patients at risk. The multifactorial nature of AKI plays an important part to that effect. A number of clinical trials have evaluated different biomarkers in plasma and urine. Some of these biomarkers (neutrophil-gelatinase-associated lipocalin-NGAL, interleukin-IL, liver-type fatty acid binding protein L-FABP, kidney injury molecule 1-KIM1) have been utilized for early identification AKI with mixed results.3 More recently, two new cell cycle arrest biomarkers; tissue inhibitor of metalloproteinase 2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7), have been isolated in different multicenter discovery and validation clinical trials and marketed in the U.S. for risk assessment of AKI.4 These are cell cycle arrest proteins synthesized and secreted in injured renal tubular cells blocking endothelial cell proliferation by kinase activation5 and affecting the bioavailability of insulin growth factors that participate in tumor suppression and cell senescence.6 These biomarkers will be able to detect moderate to severe AKI within 24h. The approval of these novel biomarkers represents a step in the search for a robust and accurate means of early diagnosis of kidney injury. We will review the biology of these biomarkers, enumerate the clinical trials performed to date to identify them and also discuss their applicability in clinical practice.
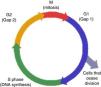
Biology of cell cycle biomarkersAs previously mentioned, cell cycle division is required for cell proliferation (Fig. 1). Cells respond to injury by repairing while entering and exiting different phases of proliferation assisted by kinases.7 Exit from the cell cycle in G1 leads to apoptosis.7 Furthermore, failure to achieve G1 cycle arrest leads to fibrosis through collagen production during the G2 mitotic phase. On the other hand, G1 cell cycle arrest, prevents division of cells with damaged DNA, permitting adequate repair.8 This is a protective mechanism designed to avoid exposure to stress and injury. If cells become arrested at the G1 or G2 phases for prolonged periods, senescence and fibrosis will ensue.9 This cell cycle is regulated by different proteins, particularly kinases, and they become activated in response to DNA cell damage in order to initiate repair. In situations of cellular stress several tumor suppressor protein will become activated, particularly P53 and P27, and will upregulate different proteins through cycling complexes (Cdk2/cyclin E and 4/cyclin D) blocking transcription and cell cycle progression in G1. The consequence will be stress at the cellular level that could translate in the synthesis of TIMP-2 and IGFBP7.10
Tissue inhibitor of metalloproteinase 2 might play a distinctive role in renal injury, triggering tubulointerstitial fibrosis as described in patients with matrix metalloproteinase mediated chronic allograft nephropathy.11 Insulin-like growth factor binding protein 7 is a member of a large family of proteins with pleiotropic effects that include tumor suppression and induction of cell senescence in cancer cells. During the repair phase of AKI, tubular interstitial fibrosis settles in, contributing to the development of chronic kidney disease (CKD) and end-stage kidney disease (ESKD). Hence, these cell cycle biomarkers, when isolated in the urine or serum, speculatively detect ischemic and or toxic injury cell stress at an earlier stage. Despite establishing a plausible connection between the up regulation of TIMP-2 and IGFBP7 and tubular cell injury through DNA damage, their biological role in AKI has not been fully elucidated.12
Discovery and validation studiesThe use of TIMP-2 and IGFBP7 has been previously validated as possible predictors of AKI on a rat model of sepsis.13 Four studies (Discovery, Sapphire, Opal, Topaz) have been previously conducted to implement the use of this biomarkers in clinical practice. The discovery study evaluated the predictability of AKI risk of 340 candidate biomarkers in critical care patients. The results showed that urine TIMP-2 and IGFBP7 perform better than other biomarkers particularly NGAL, IL-18, L-FABP, and KIM-114 (Fig. 2). Kashani et al.14 later pooled data from 3 cohort studies (Vienna, Duke, Mayo) for analysis and assembled a fourth study (Sapphire) with the idea of validating the performance of the newly discovered cell cycle arrest biomarkers against approximately 300 biomarkers. Seven hundred forty-four patients were recruited from 35 clinical sites in North America and Europe, and observed for the development of moderate to severe AKI (RIFLE/AKIN criteria stage 2–3)15,16 within 24h of intensive care unit (ICU) admission. Thirty one percent were in medical ICU, 19% were admitted with sepsis and 33% with cardiovascular complications. Primary analysis was based on area under the receiver-operating characteristics curve (AUC; graphical plot that illustrates the performance of a binary classifier with different variable discrimination thresholds plotting true positive and false positive rates) comparing TIMP-2 and IGFBP7 with other biomarkers in urine and serum. The AUC for urine TIMP-2 and IGFBP7 was significantly greater than any other biomarkers (AUC cut off 0.80 for AKI stage 2–3) (P<0.002). Studies have shown that the average significant cut-off value (sensitivity and specificity) for a biomarker is 0.70ng/ml2/1000.17 The cut off values of 0.3 and 2.0ng/ml2/1000 for TIMP-2 and IGFBP7 were developed based on the sensitivity and specificity to determine the risk for AKI. These biomarkers also showed a clear separation between AKI and non AKI conditions including chronic kidney disease.18 A sensitive of 89% and negative predictive value of 97% for the cut off of 0.3 and a specificity as well as positive predictive value of 95% and 49% respectively for the cut off of 2.0 was later confirmed in a study that involved 154 patients, 18% of them with stage II or 3 AKI (OPAL).19 Bihorac et al.20 adjudicated 420 critically ill patients to a panel of nephrologist that blindly determine if the population study had stage 2–3 AKI per KDIGO.21 A cut off value for [TIMP-2]×[IGFBP7] of 0.3 was chosen because of its high sensitivity. Results showed that 25% of patients with [TIMP-2]×[IGFBP7]>0.3 developed AKI (Fig. 3).
Area under the receiver-operating characteristics curve (AUC) for novel urinary biomarkers and existing biomarkers of acute kidney injury (KDIGO stage 2 or 3 within 12h of sample collection). The AUC for urinary [TIMP-2]×[IGFBP7] is larger than for the existing biomarkers IGFBP7, insulin-like, IL-18, interleukin-18; KIM-1, kidney injury marker-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.14
Based on the above mentioned results, the FDA (U.S. federal drug administration) allowed Astute Medical to market TIMP-2 and IGFBP7 under NephroCheck on September 5, 2014 with the intention to be used as a diagnostic tool for risk assessment of moderate to severe AKI in critically ill patients. The test was also intended to be use in conjunction with other clinical and diagnostic parameters utilized to identify AKI particularly serum creatinine and urine output.
Implementation of cell-cycle arrest biomarkers in different clinical scenariosAcute kidney injury is associated with increased risk of adverse outcomes, particularly death and end-stage renal disease. Therefore the use of biomarkers that can predict the probability of developing AKI represent an important clinical tool for AKI prevention or attenuation of renal injury. This was illustrated by a recent clinical trial of cardiac surgery patients who were identified as being at risk for AKI by TIMP-2 and IGFBP7 testing. In this randomized trial, patients in whom the KDIGO AKI management guideline bundle (optimization of volume status and hemodynamics, avoidance of nephrotoxic drugs, prevention of hyperglycemia) was applied had a lower risk for AKI than dose managed with standard care. AKI biomarkers were also prove helpful for de-escalation of care.22 Srisawat et al.23 looked at a cohort group of 76 patients from the biological markers of recovery for the kidney study (ancillary to the acute renal failure trial Network study24) and found that decreasing urinary hepatocytes growth factor and NGAL were associated with renal recovery (P=0.01 and 0.003, AUC (0.74, 95% CI)). Such studies are not yet available for cell cycle arrest markers. Previous studies have determined the predictability of cell-cycle arrest biomarkers (TIMP-2 and IGFBP7) based on different cut offs for risk assessment of kidney injury. Koyner et al.,25 after looking at the Saphire data and applying a COX proportional model adjusted for serum creatinine, demonstrated that TIMP-2 and IGFBP7 values >2.0 (AUC) were associated with 2 times the risk of dying or receiving renal replacement therapy.
The risk of acute kidney injury is particularly relevant in certain clinical scenarios. Cardiac surgery represents a situation where the renal insult could be identified at a specific point in time. The use of traditional markers in clinical practice, particularly serum creatinine, is influenced by the presence of non-renal factors like hemodilution, nutritional status and the poor correlation between serum creatinine and renal parenchymal damage. Same caveats also apply to urine output, particularly patient's volume and hemodynamic status. The above mentioned cell-cycle biomarkers have been implemented as early prediction tools in pediatric patients. Meersch et al.26 studied urinary [TIMP-2]×[IGFBP7] and other biomarkers in a pediatric population of 51 patient undergoing cardiopulmonary bypass (CPB). Primary outcome was development of AKI based on the RIFLE criteria.15 AKI occurred in 24% patients, with an appearance of [TIMP-2]×[IGFBP7] in urine samples, 4h after the surgical procedure that correlated with a 0.3mg baseline increase in serum creatinine. The AUC for urinary [TIMP-2]×[IGFBP7] was 0.85 (CI: 0.72–0.94) similar to urine NGAL AUC but higher than KIM-1. Interestingly, pediatric patients displayed higher baseline appearance of urinary [TIMP-2]×[IGFBP7] when compare with adults. Limitations of the study were a single center and small population. The same investigators evaluated similar biomarkers on an adult population undergoing CPB. In this case the AUC benefited urinary [TIMP-2]×[IGFBP7] in comparison with NGAL (AUC 0.81 versus 0.68). Cut off value for sensitivity and specificity was 0.3ng/ml2/1000. These results correlated with the ability of urinary [TIMP-2]×[IGFBP7] in diagnosing early AKI as well as renal recovery even before changes in serum creatinine.27 Dusse et al. evaluated 40 patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI) with a primary endpoint of stage 2–3 AKI identification based on KDIGO classification. At a cut off value of 0.91ng/ml2/1000, the sensitivity and specificity of [TIMP-2]×[IGFBP7] for detecting patient who developed KDIGO AKI 2–3 was 87.5 and 82.8% respectively (AUC 0.869). Also the sensitivity and specificity for detecting patients in need for renal replacement therapy in the next 72h was 100% and 80% respectively. Once more, these results confirmed that cell cycle arrest biomarkers performed better than serum creatinine determinations.28
In a paradigm shift, Saad et al.29 showed that renal vein levels of TIMP-2 and IGFBP7 were elevated in patients undergoing renal artery stenting for atherosclerotic renal artery stenosis. Values correlated inversely with percent changes in cortical-medullary renal hypoxia (measured by BOLD (blood oxygen level-dependent-MRI)). In this case the increase was associated with protection against contrast induced exposure hypoxia as part of a mechanism of “ischemic preconditioning” and resistance to develop AKI. At the end of 3 months there were no changes in single kidney GFR. Cell cycle arrest biomarkers predicted better renal outcome after contrast exposure.
Delayed graft function (DGF) represents “the Achilles tendon” of kidney transplantation and is associated with increased risk of rejection and long-term graft function loss. Pianta et al., in a proof of concept study that involved deceased donor kidney transplant recipients, demonstrated 12h post transplantation, that urinary TIMP-2 and IGFBP7 were good predictors of DGF in comparison with serum creatinine. The results suggested a plausible correlation between elevated urinary concentrations of cell cycle arrest biomarkers with tubular epithelial damage in this case due to DGF.30
Finally, Honore et al. analyzed data from a cohort of 232 patients from the previously mentioned Saphire and Topaz studies. Utilizing several cutoff values for TIMP-2 and IGFBP7, they determined that the absolute risk of AKI (cut-off value of >2.0) was 53.3% (95% CI, P<0.001).31
As mentioned above, the US Food and Drug Administration approved the point of care urinary biomarker assay (TIMP-2 and IGFBP7) (Nephrocheck, Astute Medical San Diego, CA, USA) for predicting risk of AKI. Paradoxically, Bell et al.,32 using the Nephrocheck system found no correlation between cell cycle arrest biomarkers and prediction of AKI within 12h of a renal event.
On the other hand, Pajenda et al.33 again utilizing the same system on a diverse group of patients with multifactorial AKI (recipients of a cadaveric kidney with ischemia reperfusion, congestive heart failure with cardio renal syndrome, hepatorenal and cisplatinum induced nephrotoxicity), showed that TIMP-2 and IGFBP7 in urine rise prior to any change in serum creatinine and its decline correlated with restoration of kidney function. There was a lack of correlation between high TIMP-2 and IGFBP7 levels and rise in serum creatinine particularly on patients exposed to chemotherapy agents. Apparently high urine bilirubin and albumin levels may interfere with the test.
Recently Heung et al.34 constructed receiver operating characteristics curves for 1131 patients from the Saphire14 and Topaz20 studies with AKI and diverse comorbidities like diabetes mellitus, congestive heart failure, and chronic kidney disease. The percentage of patients with CKD (mean eGFR 52ml/min) was similar between AKI groups. TIMP-2 and IGFBP7 cut off points were higher in AKI versus non-AKI patients (AUC 0.81) and 12.3% of those patients developed KDIGO stage 2–3 AKI. Patients with congestive heart failure had higher AUC values. There was no significant difference in sensitivity or specificity at different cutoffs (0.3 and 2.0ng/ml2/1000). Cell cycle arrest biomarkers performed well in the presence of chronic comorbid conditions. In stark contrast a study conducted by Hsu et al. demonstrated a lack of predictability of traditional urinary biomarkers in patients with CKD.35
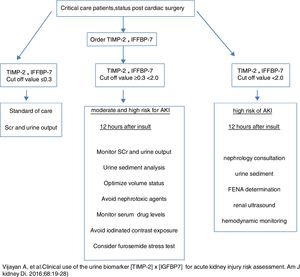
As noted, TIMP-2×IGFBP7>0.3 has a sensitivity of 92% for moderate to severe AK in 24h after the injury.36 The specificity of this cut off was only 46% with a positive predictive value of 27% (CI, 21–32%). Therefore fall positive results will appear if the test is used in low risk patient since the predictive value of the test depends on presence of the disease. Serum troponin is useful to assess early diagnosis of myocardial infarction when tested in the appropriate setting (chest pain with clinical risk factors and EKG changes). Similarly a TIMP-2×IGFBP7 value of >0.3 will have greater predictability in patients at higher risk for AKI but will be less useful in patients at low risk. For that reason these biomarkers should not be use in the outpatient setting and is not beneficial in patients with established KDIGO stage II or 3 AKI since it is unknown how long elevations will persist and predict worsening of AKI or kidney recovery. Appropriate patient population should be adults more than 21 years of age, with at least one other risk factor for AKI undergoing cardiac bypass or other major high risk surgery and presence of sepsis in the ICU setting. Obviously these biomarkers should not be considered substitute for serum creatinine measurements, urine output and urine sediment evaluation. Daily routine measurements are not recommended with the exception of patient with changes in clinical situation. Hyperbilirubinemia and albumin levels interfere with the test and clinicians should be aware of this limitation. The combination of urine sediment and TIMP-2×IGFBP7 could potentially increase the threshold for early detection of AK. A practical approach to the use of these biomarkers should be considered (Fig. 3).
We have shown the predictability of cell cycle arrest biomarkers, particularly TIMP-2 and IGFBP7, as tools for early detection of AKI in certain clinical scenarios. The test is designed to identify patients at risk for AKI at early stages. Some studies are contradictory on their results. We still have to elucidate how to implement these biomarkers on a day to day clinical practice. However, a recent clinical trial has demonstrated the ability to impact outcomes by identifying patient is at high risk for AKI using cell cycle arrest markers.22
ConclusionThe use of serum and urine biomarkers to detect early AKI derives from the need to implement therapeutic measures with the goal of preventing irreversible renal damage. These biomarkers are intended to be use in a similar fashion as cardiac troponins. Like any other test, they are subject to sensitivity and specificity. False-positive result will appear if the test is used in patients that have low risk for AKI.
Cycle arrest biomarkers have a high sensitivity but low specificity and predictive value depends on the pretest probability of the disease. They should be use as an aid to assess the risk of moderate-to-severe acute kidney. They should also be incorporated with clinical and diagnostic parameters in accordance with current clinical practice (serum creatine, urine sediment, hemodynamics).37
The optimal way to use these biomarkers remains uncertain at the present time.35 The costs for the NephroCheck test are higher than serum creatinine which is the current gold standard for determination of renal function. On the other hand, the presence of AKI represents a personal and financial burden to health care. Furthermore, early recognition of patients at risk for AKI would be an important step in order to decrease the incidence as severity of renal injury and prevent dire consequences particularly the development of end-stage renal disease.
The findings from Meersch, Zarbock et al. clinical trial allows for the use of cell cycle arrest markers in order to prevent AKI by identifying high risk patients in whom aggressive preventive measures could be implemented.20
Preemptively maintaining proper hemodynamics, avoiding nephrotoxin agents, and hydration depending on volume status are measures that could be applied at an earlier stage, once increased cell cycle arrest biomarkers levels are determined, particularly within the stipulated cut-off values.
In conclusion, the discovery of these biomarkers represents a step forward toward early recognition of AKI with the purpose of implementing therapies that will avoid the need for renal replacement therapy and prevent the development of CKD and ESRD in our critical care population. Additional trials are needed in order to fully characterize their niche in clinical practice.
Conflict of interestsThe authors have no conflict of interest.







![Area under the receiver-operating characteristics curve (AUC) for novel urinary biomarkers and existing biomarkers of acute kidney injury (KDIGO stage 2 or 3 within 12h of sample collection). The AUC for urinary [TIMP-2]×[IGFBP7] is larger than for the existing biomarkers IGFBP7, insulin-like, IL-18, interleukin-18; KIM-1, kidney injury marker-1; L-FABP, liver fatty acid-binding protein; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2.14](https://static.elsevier.es/multimedia/02116995/0000003800000004/v1_201807200928/S0211699517302291/v1_201807200928/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)