The irreversible progression of autosomal dominant polycystic kidney disease (ADPKD) to end-stage renal disease (ESRD) is delayed by tolvaptan. Therefore, we aim to systematically estimate and evaluate the efficacy and safety of tolvaptan in the treatment of ADPKD.
MethodsTwo reviewers independently searched all published randomized controlled trials studies in PubMed, EMBASE, Web of Science and Cochrane databases, extracted data, assessed bias risk and rated the quality of evidence. Data were analyzed by the RevMan software.
ResultsWe identified 8 trials including 2135 patients. Both of the decline of estimated glomerular filtration rate (eGFR) [MD=1.89, 95% CI (0.74, 3.04), P=0.001] and total kidney volume (TKV) [MD=−3.32, 95% CI (−4.57, −2.07), P<0.001] were delayed in tolvaptan group compared with placebo group in ADPKD patients. The use of tolvaptan delayed TKV progression in the different-month subgroups [MD=−69.99, 95% CI (−91.05, −48.94), P<0.001]. Tolvaptan reduced renal pain [RR=0.66, 95% CI (0.54, 0.81), P<0.001] and hematuria events [RR=0.55, 95% CI (0.41, 0.74), P<0.001] in ADPKD patients. However, the prevalence of thirst [RR=2.75, 95% CI (2.34, 3.24), P<0.001] and nocturia events [RR=3.01, 95% CI (1.27, 7.11), P=0.01] were increased in tolvaptan group. There is no significant difference of hypertension events [RR=0.92, 95% CI (0.82, 1.03), P=0.13] in tolvaptan group compared placebo group.
ConclusionsThis meta-analysis suggests that tolvaptan may improve clinical progression in patients with ADPKD without significantly increasing the risk of adverse reactions.
La progresión irreversible de la enfermedad renal poliquística autosómica dominante (ERPAD) a enfermedad renal en etapa final (ESRD) es demorada por tolvaptan. Por tanto, nuestro objetivo fue estimar y calcular sistemáticamente la eficacia y seguridad de tolvaptan en el tratamiento de ERPAD.
MétodosDos revisores buscaron de manera independiente todos los estudios publicados sobre ensayos controlados aleatorizados en las bases de datos de PubMed, Embase, Web of Science y Cochrane, extrayendo datos, evaluando el riesgo de sesgo y calificando la calidad de la evidencia. Los datos fueron analizados utilizando el software RevMan.
ResultadosIdentificamos ocho ensayos, que incluyeron 2.135 pacientes. Tanto la reducción de la tasa de filtración glomerular estimada (eGFR) [MD=1,89, IC 95% (0,74, 3,04), p=0,001] como el volumen renal total (VRT) [MD=−3,32, IC 95% (−4,57, −2,07), p<0,001] se demoraron en el grupo tolvaptan, en comparación con el grupo placebo en los pacientes con ERPAD. El uso de tolvaptan demoró la progresión del VRT en los subgrupos de diferentes meses [MD=−69,99, IC 95% (−91,05, −48,94), p<0,001]. Tolvaptan redujo el dolor renal [RR=0,66, IC 95% (0,54, 0,81), p<0,001] y los episodios de hematuria [RR=0,55, IC 95% (0,41, 0,74), p<0,001] en los pacientes con ERPAD. Sin embargo, la prevalencia de episodios de sed [RR=2,75, IC 95% (2,34, 3,24), p<0,001] y nocturia [RR=3,01, IC 95% (1,27, 7,11), p=0,01] se incrementó en el grupo tolvaptan. No existe diferencia significativa en cuanto a episodios de hipertensión [RR=0,92, IC 95% (0,82, 1,03), p=0,13] en el grupo tolvaptan, en comparación con el grupo placebo.
ConclusionesEste metaanálisis sugiere que tolvaptan puede mejorar la progresión clínica en los pacientes con ERPAD, sin incrementar significativamente el riesgo de reacciones adversas.
Autosomal dominant polycystic kidney disease (ADPKD) is a common single-gene inherited kidney disease, which is more common in middle-aged onset. Most ADPKD is caused by PKD1 gene (85% of cases) or PKD2 gene mutation.1 Within the last 5 years, various other genes could be identified in the small percentage of patients without PKD1 or 2 mutations: mutations in GANAB cause generally mild cases of ADPKD and polycystic liver disease,2 patients with DNAJB11 variants have non-enlarged or atrophic kidneys with interstitial fibrosis and mutations in ALG9 also cause polycystic kidney and/or liver disease, but the further characterization of disease progression in the latter two is still lacking.3,4 What these genes have in common is that they are thought to lead to inefficient maturation and transportation of membrane or secreted proteins, with a specific dose sensitivity for PC1, leading to kidney or liver cysts.5 ADPKD is characterized by the continuous development and growth of cysts. This results in progressive renal enlargement with hypertension, abdominal fullness and pain, episodes of cyst bleeding, gross hematuria, kidney calculi, cyst infection, and reduced quality of life (QOL)6,7 while approximately 50% of patients with ADPKD irreversibly progress to end-stage renal disease (ESRD) by the age of 60, requiring dialysis and kidney transplantation.8 This not only seriously affects the quality of life of patients, but also greatly wastes social resources. Therefore, exploring effective therapeutic measures for ADPKD has become a hot research topic in this field.
Tolvaptan is a selective vasopressin-2-receptor (V2R) antagonist, which is mainly used for the treatment of high-volume and normal-volume hyponatremia, including heart failure, liver cirrhosis and syndrome of inappropriate antidiuretic hormone secretion. In recent years, several large-scale international clinical randomized controlled trials have shown that tolvaptan can effectively inhibit the growth of renal cysts in patients with ADPKD and delay the deterioration of renal function. Many countries, including the United States, have approved the use of tolvaptan in the treatment of rapidly progressive adult ADPKD patients.9–14 However, Gross et al.15 have revealed that tolvaptan is associated with the deterioration of GFR function in patients with ADPKD, and tolvaptan can aggravate GFR by 3–5%. In addition, some studies have suggested16 that tolvaptan in the treatment of ADPKD can cause severe adverse events such as pulmonary embolism. Therefore, the safety of tolvaptan in the treatment of ADPKD is also questioned. Therefore, we intended to conduct a systematic evaluation of the efficacy and safety of tolvaptan in patients with ADPKD in order to provide evidence-based evidence for the treatment of ADPKD with tolvaptan.
Materials and methodsLiterature searchWe searched the online databases PubMed, Web of Science, Cochrane Library, and Embase for related research until January 2022. The following search terms were used: “TKV,” “eGFR,” “ADPKD,” “polycystic kidney disease and tolvaptan,” “kidney disease,” as well as “Vasopressin v2 receptor antagonist”. The method of combining subject words and free words was adopted flexibly for retrieval, and the corresponding settings were made according to the characteristics of different databases, with “Polycystic Kidney Disease and Tolvaptan” limited as the key word. Details of search strategy are shown in Table 1.
General information of included studies.
| Study | Year | Country | Research type | Number of cases (T/P) | Sex (male/female) | Age, years (SD) | Intervention | Follow-up duration | Reported outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | P | |||||||||||
| Higashihara18 | 2011 | America | RCT | 51/102 | T=17/34 | P=34/68 | T=42±8.8 | P=42±9.4 | Tolvaptan 15/15, 45/15, 90/30mg once daily, with gradual weekly increases of 45/15, 90/30mg if tolerated | Placebo | 3yr | |
| Torres9 | 2012 | America | RCT | 961/484 | T=495/466 | P=251/233 | T=39±7 | P=39±7 | Tolvaptan 45/15, 60/30, 90/30mg once daily as tolerated with a gradual increase of 60/30mg, 90/30mg per week | Placebo | 3yr | |
| Satoru Muto23 | 2015 | Japan | RCT | 118/59 | T=59/59 | P=35/24 | T=38.7±6.1 | P=40.4±5.6 | Tovaptan 60/120mg, once daily | Placebo | 1yr | |
| Casteleijn19 | 2017 | Netherlands | RCT | 961/484 | T=495/466 | P=251/233 | T=39±7 | P=39±7 | Tolvaptan 45/15, 60/30, 90/30mg once daily, with gradual weekly increases of 60/30mg, 90/30mg if tolerated | Placebo | 3yr | |
| Torres24 | 2016 | America | RCT | 958/481 | T=494/464 | P=249/232 | T=38.6±6.3 | P=39.3±6.6 | Tolvaptan 45/15, 60/30, 90/30mg once daily, with gradual weekly increases of 60/30mg, 90/30mg if tolerated | Placebo | 3yr | |
| Perrone22 | 2020 | America | RCT | 134/43 | T=73/61 | P=20/23 | T=34±8.5 | P=33.9±8.1 | Tolvaptan 50mg/once daily | Placebo | 8wk | |
| Silvia Lai21 | 2020 | Italy | RCT | 10/26 | T=7/3 | P=15/11 | T=36.7±9.7 | P=42.5±7.0 | Tolvaptan 45/15, 60/30, 90/30mg once daily, with gradual weekly increases of 60/30mg, 90/30mg if tolerated | Placebo | 1yr | |
| Shigeo Horie20 | 2021 | Japan | RCT | 92/55 | T=48/44 | P=33/22 | T=38.9±11.9 | P=40.5±5.6 | Tolvaptan 60/30mg, 90/60mg once daily, with a gradual increase of 90/30mg weekly if tolerated | Placebo | 3yr | |
The following criteria were used for study inclusion and selection: (i) study design: a randomized controlled trial; (ii) type of participants: adults (age≧18 years) with ADPKD; (iii) intervention: tolvaptan; (iv) controlled studies: placebo or no treatment; (v) outcome assessed: the main outcome was the delay of eGFR and TKV; secondary outcomes were the primary outcome measures: renal pain, hematuria, proteinuria, hypertension, and incidence of nocturia events.
The exclusion criteria were as follows: (i) animal research and cell experimental research; (ii) repeated literature by the same research group, and select the latest report with the most complete data; (iii) review literature, case studies, guidelines, systematic analysis and other non-RCT literature; (iv) the data provided by the research is incomplete or missing.
Study eligibility and selectionLiterature screening, data extraction and quality evaluation were conducted independently by two researchers. The final results were cross checked by the two researchers. If there was no agreement, a third-party expert would be consulted and the revised risk assessment tool for randomized trial bias developed by Cochrane Collaboration17 would be used to assess all potential relevant deviations. Each study outcome was assigned as “low risk of bias,” “some concerns,” or “high risk of bias.” Extraction of literature data: (i) basic information of the literature (including publication, first author, year and region of publication); (ii) study type is RCT; (iii) the types of subjects included in the analysis, the total number of cases and the frequency of polycystic kidney outcomes in each group; (iv) the time, dose and type of tolvaptan used.
Data synthesis and analysisThe eGFR, TKV, treatment duration, and different doses of the drug changes were expressed as the mean difference (MD) of the 95% confidence intervals. For adverse events, risk ratios (RR) and 95% confidence intervals were calculated using raw data, followed by a meta-analysis using a random-effect model that was selected to explain the expected heterogeneity in study design, study drug timing and dose, and some outcome definitions, all using software Review Manager version 5.3. Although all studies strictly followed the inclusion and exclusion criteria, heterogeneity was inevitable. Heterogeneity was assessed using I2 statistics (I2≧50% or the corresponding P value<0.10 indicated statistically significant heterogeneity between studies), and as fewer than 10 studies were identified, publication bias could not be assessed visually in the funnel plot. A P value≦0.05 was considered statistically significant. All P-values were statistically significant set to 0.05 with a confidence interval of 95%. This study is registered at the PROSPERO registry under reference number CRD42022328034.
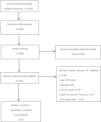
ResultsStudy selectionAccording to the established retrieval strategy, 1301 relevant studies were preliminarily retrieved. 818 articles were excluded by removing duplicates. Forty-five potentially eligible studies were identified by screening the remaining headings and abstracts, and exclusion of the review animal studies categories, and studies and trials that did not meet our inclusion criteria were excluded after further reading. After screening, 8 studies met our inclusion criteria (Fig. 1). Table 1 summarizes the characteristics of the included trials.
Study characteristicsThe characteristics of the included trials are summarized in Table 1. 8 RCT studies were enrolled, totaling 2135 patients, including 1366 cases in the experimental group and 769 cases in the control group. In the 8 studies, the experimental group was tolvaptan and the control group was placebo. Doses of tolvaptan ranged from 15mg to 90mg per day and were gradually increased weekly if tolerated. The follow-up period ranged from 8 weeks to 3 years, in all including studies, to evaluate changes in eGFR, TKV, and some adverse events or complications (Table 1). Among them, regarding the change of therapeutic effect of TKV, as mentioned by the Eiji Higashihara et al.18, 51 subjects (81%) completed 3 years of treatment with tolvaptan; initial baseline TKV (1422ml for placebo and 1635ml for tolvaptan). During the overall three-year period, total renal volume increased by 5.8%/year in the placebo group and 1.7%/year in the tolvaptan group. The work of Casteleijn et al.19 mentioned that the TKV volume had a baseline of 1668ml in the placebo group and 1705ml in the tolvaptan group, and that tolvaptan, a vasopressin V2 receptor antagonist, increased the total renal volume by 2.8% [95% CI (2.5–3.1)] per year in the tolvaptan group and 5.5% [95% CI (5.1–6.0)] per year in the placebo group over the 3-year period, P<0.001.
Risk of bias and quality assessmentThe overall research quality is medium to high, and there are some potential deviations. The deviation risk is evaluated based on several fields, including allocation deviation, loss deviation, and detection deviation and reporting deviation. The evaluation results are given in Table 2 by using the Cochrane deviation risk tool. Most studies have not disclosed enough hidden details about sequence generation or allocation. The use of other treatment methods, I.E. the control of blood pressure and low back pain, has not been explicitly reported in some studies, thus indicating that there are “high risks” and “some concerns” in the field of deviation (Table 2).
Risk of bias assessment conducted using the Cochrane risk of bias tool of the included studies.
| Author | Allocation bias | Performance bias | Attrition bias | Detection bias | Reporting bias |
|---|---|---|---|---|---|
| Higashihara et al.18 | Low | Low | Low | Some concern | Low |
| Torres et al.9 | Low | Low | Low | Low | Low |
| Satoru Muto et al.23 | Low | Low | Low | Some concern | Low |
| Casteleijn et al.19 | Low | Low | Some concern | Some concern | Low |
| Torres et al.24 | Low | Low | Low | Low | Low |
| Perrone et al.22 | Low | Low | Some concern | Low | Low |
| Silvia Lai et al.21 | Low | Low | Some concern | Some concern | Low |
| Shigeo Horie et al.20 | Low | Low | Low | Some concern | Low |
As shown in Fig. 2, four studies18–21 in patients with ADPKD reported rates of change in eGFR and two studies18,19 reported annual rates of change in TKV. Analysis of pooled data revealed heterogeneity between eGFR and TKV as compared to controls, a randomized-effect model for meta-analysis, and glomerular filtration rate [MD=1.89, 95% CI (0.74, 3.04), P=0.001, I2=59%] (Fig. 2a) and total kidney volume [MD=−3.32, 95% CI (−4.57, −2.07), P<0.001, I2=70%] (Fig. 2b).
Forest plots of the included randomized controlled trials. Included patients had ADPKD: (a) showing a difference in estimated glomerular filtration rates between tolvaptan and placebo; (b) showing a difference in annual rate of change of renal volume rates between tolvaptan and placebo. 95% CI: 95 confidence interval; df: degrees of freedom; IV: inverse variance.
As shown in Fig. 3, five studies9,19,22–24 have reported the treatment duration of tolvaptan in the treatment of ADPKD patients. Due to large heterogeneity, a random effect model was used for meta-analysis. We divided the treatment duration of tolvaptan into three time periods, and analyzed the time periods in monthly subgroups. Compared with the control group, the overall treatment duration [MD=−69.99, 95% CI (−91.05, −48.94), P<0.001, Fig. 3] was statistically significant, with a one-month treatment effect [MD=−35.68, 95% CI (−70.08, −1.29), P=0.04, Fig. 3]; two-month treatment effect [MD=−72.69, 95% CI (−139.24, −6.10), P=0.03, Fig. 3]; march treatment effect [MD=−107.49, 95% CI (−211.18, −3.81), P=0.04, Fig. 3].
Forest plots of the included randomized controlled trials. Included patients had ADPKD: this figure shows the overall efficacy of tolvaptan versus placebo treatment duration, with subgroups defining efficacy by month of treatment. 95% CI: 95 confidence interval; df: degrees of freedom; IV: inverse variance.
As shown in Figs. 4 and 5, five studies9,19,22–24 reported the adverse reactions of patients taking tolvaptan in the experimental group and placebo in the control group. The adverse events in 1876 subjects included renal pain, hematuria, thirst, nocturia, hypertension, etc. The statistical analysis of adverse event data is as follows: the incidence of renal pain [RR=0.66, 95% CI (0.54, 0.81), P<0.001, I2=0] (Fig. 4a) and hematuria [RR=0.55, 95% CI (0.41, 0.74), P<0.001, I2=0] (Fig. 4b) in the treatment group were lower than that in the control group, while in thirst [RR=2.75, 95% CI (2.34, 3.24), P<0.001, I2=0] (Fig. 4c) and nocturia [RR=3.01, 95% CI (1.27, 7.11), P=0.01, I2=60%] (Fig. 4d) were higher than those of the control group. There was no heterogeneity in the incidence of hypertension in the composite data analysis, and the treatment group [RR=0.92, 95% CI (0.82, 1.03), P=0.13, I2=0%] (Fig. 5a) compared with the control group indicated that there is no statistical significance in the adverse events of hypertension in the treatment group.
Forest plots of the included randomized controlled trials. Included patients had ADPKD: (a) shows the difference between tolvaptan and placebo in estimating the change in the rate of renal pain; (b) shows the difference between tolvaptan and placebo in estimating the change in the rate of hematuria; (c) shows the difference between tolvaptan and placebo in estimating the change in the rate of thirst; (d) shows the difference between tolvaptan and placebo in estimating the change in the rate of nocturia. 95% CI: 95 confidence interval; df: degrees of freedom; M-H: Mantel-Haenszel.
Forest plots of the included randomized controlled trials. Included patients had ADPKD: (a) shows the difference between tolvaptan and placebo in estimating change in the rate of the hypertension; (b) shows the difference between tolvaptan and placebo in estimating changes in treatment with different doses of the drug. 95% CI: 95 confidence interval; df: degrees of freedom; M-H: Mantel-Haenszel.
As shown in Fig. 5b, two studies18,22 have reported to compare the different doses of the drug to reduce the risk of renal pain in patients with ADPKD. Meta-analysis was conducted for comprehensive data and there was no heterogeneity. There was no statistical significance in the high-dose group when compared with the low-dose group [MD=1.26, 95% CI (0.65, 2.44), P=0.50, I2=0] (Fig. 5b).
DiscussionEvidence-based medicine needs to understand the heterogeneity of results by considering the integrity of evidence, and guide clinical practice with reliable evidence. In this study, we systematically analyzed the effects of tolvaptan on patients with ADPKD. The results of this study showed that: (i) the use of tolvaptan effectively slowed the decline of eGFR and TKV. Efficacy analyses for ADPKD patients in the different months remained consistent across subgroups, delaying the progression of TKV in the different months, and not worsening efficacy over time. Rather, efficacy might have been better. However, due to the scarcity of available data and some heterogeneity, this finding should be considered exploratory rather than definitive. (ii) After the meta-analysis of the efficacy of tolvaptan in different doses, there was no statistically significant difference between the doses. However, due to the small amount of literature currently studied, statistical evidence does not show that there is no difference between low dose and high dose of tolvaptan. In the future, more high-quality articles and expanded sample size can be considered, and the difference between the two groups can be counted again. Perhaps the comparison between two groups of measures has a statistical difference. (iii) Statistical analysis was performed on adverse reaction events in the tolvaptan experimental group. The adverse reaction events of renal pain and hematuria in the experimental group were less than those in the control group, but the number of adverse events such as thirst and nocturia was greater than that in the control group. In terms of improving the progression of ADPKD, the adverse reactions of thirst and nocturia can be completely tolerated, at the same time, it is also because the medicinal mechanism of tolvaptan determines that: Tolvaptan was a selective arginine vasopressin (AVP) V2 receptor antagonist. Vasopressin binds to V2-type receptors in the distal tubules and collecting ducts, promotes urine concentration, inhibits bulbar feedback, and promotes sodium salt reabsorption. The mechanism of tolvaptan is to antagonize the binding of vasopressin to V2 receptor, so it can inhibit urine concentration, stimulate tubuloglomerular feedback, and inhibit sodium salt reabsorption.25–27 Therefore, it can also be demonstrated that the adverse reactions of tolvaptan are part of the drug mechanism, but its adverse reactions are acceptable after the larger efficacy in alleviating the progression of ADPKD. Among all the adverse events, pulmonary embolism is undoubtedly the most serious. In general, when vasopressin binds to vasopressin V2 receptor, cAMP is activated and vascular endothelial cells release von Willebrand factor, both of which may lead to an increase in the clotting cascade.28 Tovaptan inhibits the release of von Willebrand factor by vascular endothelial cells through its antagonism against vasopressin V2 receptor, and may reduce the hemostatic ability.29 Therefore, administration of Tovaptan to patients with ADPKD accompanied by dehydration may be at risk of pulmonary embolism.16
So how does tolvaptan alleviate the progression of ADPKD? The epidemiological survey of ADPKD affects 12.5 million people of all ethnic groups worldwide, accounts for up to 10% of patients with end stage renal disease (ESRD), and constitutes a major public health burden.30 Most ADPKD of patients begin to suffer from a series of clinical symptoms after middle age, which can affect multiple systems of the whole body.31,32 Tolvaptan was a selective arginine vasopressin (AVP) V2 receptor antagonist and it can inhibit the production of cyclic AMP (cAMP) induced by AVP, reduce protein kinase R-like ER kinase/extracellular regulated protein kinases (pERK/ERK) activity, and inhibit the proliferation of cyst cells in patients with ADPKD. Inhibiting AVP induced creatinine secretion limits the expansion of existing cysts and allows net fluid absorption to reduce cyst size and TKV, thereby slowing cyst growth in ADPKD.33–35
The advantages of this study lie in: (i) compared with other studies, the literature has been updated, the sample size is larger, and the test efficiency is improved; (ii) there was a significant benefit of taking tolvaptan from the first year [MD=−35.68, 95% CI (−70.08, −1.29), P=0.04, Fig. 3] and continuing to the third year [MD=−107.49, 95% CI (−211.18, −3.81), P=0.04, Fig. 3], which can further provide clues for clinical treatment according to literature published by Torres.9 The therapeutic effect of ADPKD does not appear until at least one year after administration, and it can alleviate the increase in total renal volume and the decrease in renal function after three years of administration. The above results are consistent with the results of this study, suggesting that long-term high-dose treatment is more conducive to the treatment of ADPKD.
This study has the following deficiencies: (i) there are certain limitations in this study. First, the literature on the treatment of ADPKD with tolvaptan is limited, except for some studies with low quality and incomplete data. The number of studies finally included in this study is only 8. In the future, we can consider including more high quality studies to expand the sample size to make the results more convincing. (ii) The heterogeneity of adverse reactions in the results is high, which may be due to the inconsistent experimental design of included studies, the differences in the dosage and method of tolvaptan administration, the follow-up questions, and the differences in the selection of the control group. In addition, the research subjects received related interventions in the hospital, and their environment might also have a certain impact on the intervention effect.
ConclusionsCompared with the control group, tolvaptan can delay the progression of eGFR and TKV, suggesting that it may have a protective effect on the kidneys of patients with CKD. In addition, the fact that tolvaptan lasts from the first year to the third year may suggest that a longer period of tolvaptan may be more beneficial for the treatment of ADPKD, and tolvaptan is now an important option for the treatment of patients with ADPKD.
FundingThis study was supported by Scientific Research Project of Education Department of Hubei Province (grant no. B2017024) and the Natural Science Foundation of Yichang City (grant no. A20-2-002).
Conflict of interestsThe authors declare no conflict of interest.