Hyperkalemia is a common electrolyte disorder frequently observed in patients with chronic kidney disease (CKD).1 Once potassium (K+) ions are absorbed from the daily food intake, they are predominantly distributed in the intracellular space than in the extracellular space.1 Under physiological conditions, approximately 90% of the dietary K+ ions are excreted into the urine, while the remaining 10% are excreted into the feces. The prompt management of hyperkalemia is to temporarily shift the extracellular K+ ions into the intracellular space by insulin.1 However, to fundamentally treat hyperkalemia, the redundant K+ ions have to be eliminated from the body, either by the use of diuretics to facilitate the renal K+ excretion or by performing emergent hemodialysis.1
In the kidneys, two populations of K+ channels are expressed in the apical membrane of the collecting duct principal cells.2 These channels, such as the renal outer medullary K+ channels (ROMK or Kir1.1) and large-conductance calcium-activated potassium channels (BK or Kca1.1), play major roles in excreting K+ ions into the urine. Both K+ channels are directly stimulated by aldosterone and also by the decrease in the luminal K+ concentration and the increase in the distal sodium (Na+) delivery.2 Of note, BK channels are additionally stimulated by the increased renal tubular flow,3 since this elevates the intracellular calcium (Ca2+) concentration, which is the primary trigger of the channel activation. In patients with CKD, due to the renal tubular damage or dysfunction,1 these K+ channels are frequently disabled. Consequently, the impaired renal K+ excretion leads to the much higher prevalence of hyperkalemia than in the general population.1
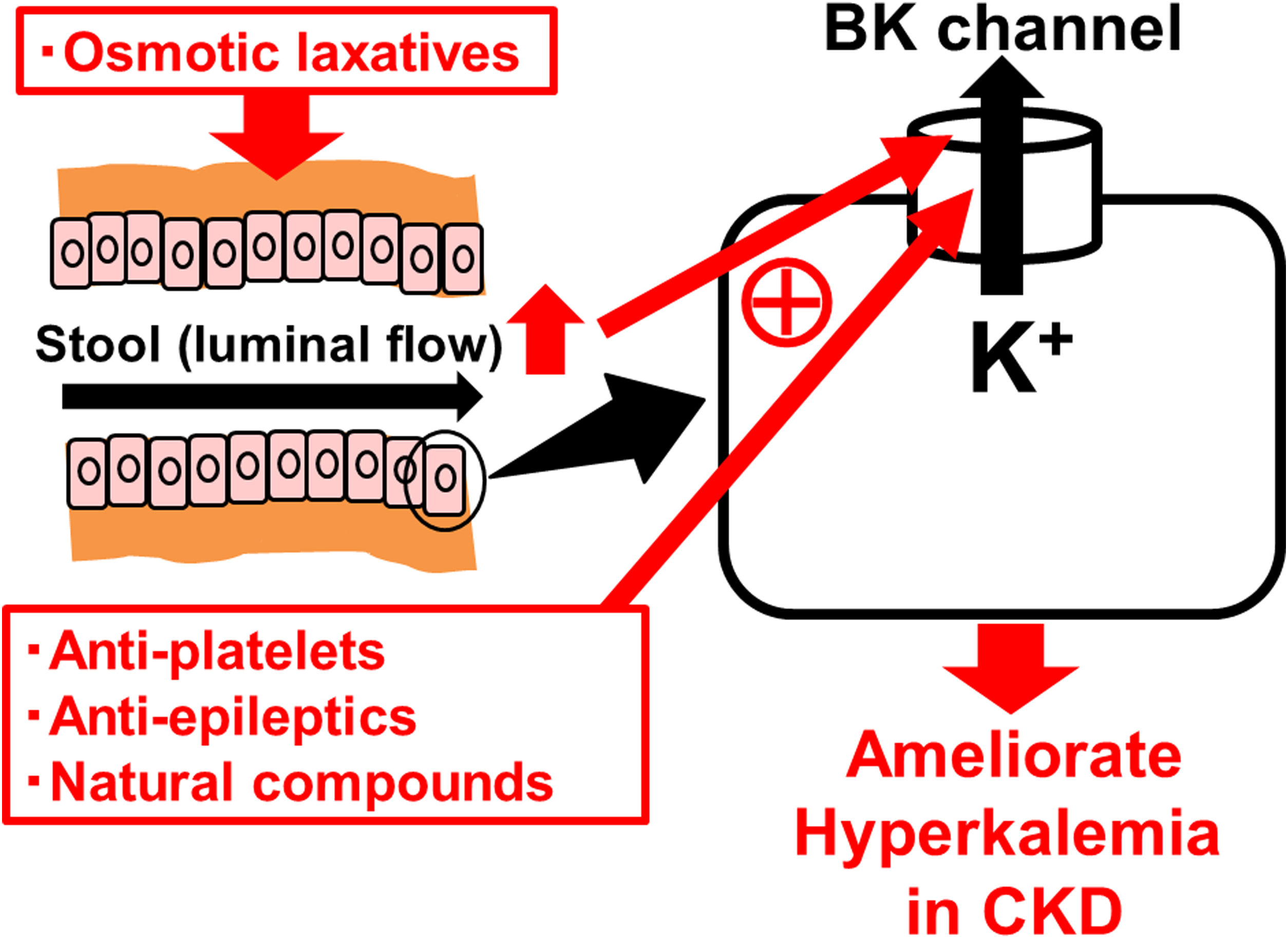
Similar to the renal collecting ducts, BK channels are also expressed in the apical membrane of colonic enterocytes and play a major role in excreting K+ ions into the feces4 (Fig. 1). In diseases that cause secretory diarrhea, such as ulcerative colitis, enteric infections, villous adenoma, BK channels are over-expressed or over-activated in the colonic epithelial cells,4 resulting in the increased fecal K+ losses. In CKD, to compensate for the decreased renal K+ excretion, its fecal excretion was alternatively increased.5 In clinical studies, since colonic BK channels were actually over-expressed in patients with advanced CKD or end-stage renal disease (ESRD),5 these channels were thought to be primarily responsible for the increased fecal K+ excretion. Therefore, drugs that directly stimulate the colonic BK channels would be effective in the treatment of hyperkalemia in CKD.
BK channels in colonic enterocytes as the therapeutic target for hyperkalemia in chronic kidney disease (CKD). BK channels are expressed in the apical membrane of colonic enterocytes and play a major role in excreting K+ ions into the feces. Already marketed drugs, such as anti-platelets (cilostazol) and anti-epileptics (zonisamide), or natural compounds, such as estradiol, omega-3 docosahexaenoic acid (DHA) and resveratrol, increase the BK channel activity. Osmotic laxatives, which increase the intestinal luminal flow, also activate the BK channels and thus ameliorate hyperkalemia in CKD.
So far, several BK channel openers, including NS1619, NS11021 and BMS204352, have newly been synthesized.6 Additionally, recent studies revealed that already marketed drugs, such as anti-platelets (cilostazol) and anti-epileptics (zonisamide)6 or natural compounds, such as estradiol, omega-3 docosahexaenoic acid (DHA) and resveratrol,6,7 also increase BK channel activity (Fig. 1). Besides the kidney and distal intestine, BK channels are also expressed in the cardiovascular system and central nervous system.6 Therefore, in using BK channel openers, the adverse effects on these organs need to be carefully monitored. However, compared to the highly selective, newly synthesized BK channel openers, the already marketed drugs or natural compounds could be used more harmlessly, because they have commonly been used in clinical practices or consumed in daily diets for longer periods of time. To increase the efficacy of these drugs or compounds after orally administered, they need to target the colonic BK channels as specifically as possible. In this regard, recently developed “drug delivery systems” that effectively reach the colon may be beneficial.8 They include pH sensitive system, microbially triggered system, prodrugs, polysaccharide-based system, timed-release system, osmotically controlled drug system and pressure-dependent release system.8
In CKD patients, chronic constipation is one of the risk factors for developing hyperkalemia,9 since it mechanically disturbs the fecal K+ excretion. However, dissolving the constipation by probiotics alone did not ameliorate hyperkalemia in these patients. One of the commonly used laxatives, bisacodyl, is also a stimulator of BK channels,10 since it pharmacologically activates a cyclic adenosine monophosphate (cAMP). In CKD patients with hyperkalemia, the treatment with bisacodyl not only improved constipation, but also increased fecal K+ excretion and actually ameliorated hyperkalemia.10 Among laxatives, osmotic laxatives, such as magnesium hydroxide, polyethylene glycol, sorbitol and lactulose, exert their properties by drawing water into the colon and allowing easier and faster passage of the stool. In addition to the passive paracellular K+ excretion,10 such-induced increase in the intestinal luminal flow would activate the colonic BK channels (Fig. 1), similarly to the increased renal tubular flow that activates the renal BK channels.3 Given such pharmacological properties, the osmotic laxatives, which increase fecal K+ excretion through the activation of colonic BK channels, would also be beneficial in the treatment of hyperkalemia in CKD patients.
Conflict of interestNone declared.
This work was supported by the Salt Science Research Foundation, No. 2218 to IK.