Peritonitis remains one of the main complications of peritoneal dialysis (PD) and one of the main reasons for abandoning this treatment and switching to hemodialysis. It also accounts for considerable mortality and hospitalization among PD patients. Most cases of peritonitis related to PD result from the contamination caused by the poor management of the Tenckhoff catheter by the patient or care-provider. The most frequently associated agents are coagulase-negative Staphylococcus and Staphylococcus aureus. However, Gram-negative bacteria and fungi may also be the cause of peritonitis.1Salmonella is an intracellular pathogen member of the Enterobacteriaceae family, and it is an extremely rare agent causing peritonitis (0.15%),2 with the particularity of being an extremely complicated organism to eradicate,3 and in most of the cases described, it was necessary to remove the Tenckhoff catheter. Literature is scarce on the ideal therapeutic approach.
We report a case of a 59-year-old male patient with arterial hypertension, atrial fibrillation, and end-stage renal disease secondary to chronic interstitial nephritis on automated peritoneal dialysis (APD) for eight months. He was regularly followed without any registration of complications and good dialysis efficacy. He presented to the emergency department with a 2-day history of intense diffused abdominal pain, associated with vomiting and watery diarrhea. On physical examination, blood pressure was 157/81mmHg, heart rate was 80/min, the tympanic temperature was 37.0°C and the abdomen was globally painful on palpation with signs of peritoneal irritation.
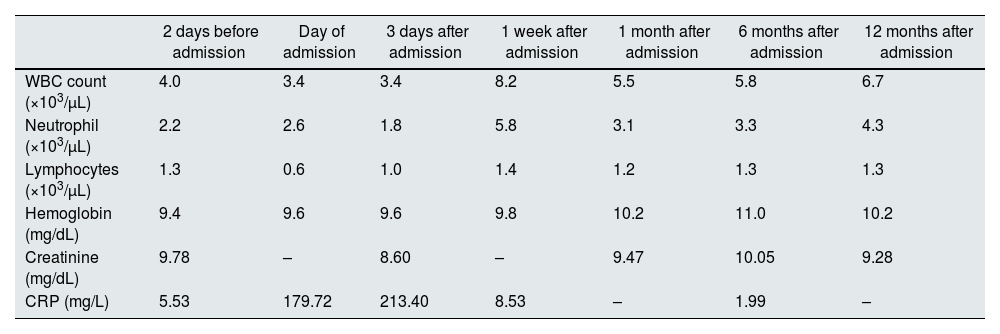
The peritoneal drainage fluid was cloudy in appearance, with 9376/mm3 cells, and predominance of polymorphonuclear cells. Complete blood count revealed 3600/μL leukocytes with 2600/μL neutrophils and 600/μL lymphocytes. Blood chemistry showed normal hepatic function and pancreatic enzymes and elevated C-reactive protein (CRP) of 179mg/L. Urgent abdominal computed tomography excluded secondary peritonitis. The patient was hospitalized at the Nephrology department and started empiric antibiotic with intraperitoneal gentamicin and vancomycin after a single intravenous dose of gentamicin 80mg and vancomycin 1000mg. Effluent culture revealed a Salmonella of the serogroup C2 sensible to gentamicin. He denied any recent intake of raw meat, poultry, eggs, or unpasteurized milk. On the 4th day of hospitalization, after a significant clinical and analytical (Table 1) improvement, he was discharged, maintaining intraperitoneal gentamicin, for a total of 21 days. To date, after 12 months follow-up, there has been no need for removal of the Tenckhoff catheter, and there are no signs of recurrence of peritonitis to date.
Progress of laboratory data from study patient.
| 2 days before admission | Day of admission | 3 days after admission | 1 week after admission | 1 month after admission | 6 months after admission | 12 months after admission | |
|---|---|---|---|---|---|---|---|
| WBC count (×103/μL) | 4.0 | 3.4 | 3.4 | 8.2 | 5.5 | 5.8 | 6.7 |
| Neutrophil (×103/μL) | 2.2 | 2.6 | 1.8 | 5.8 | 3.1 | 3.3 | 4.3 |
| Lymphocytes (×103/μL) | 1.3 | 0.6 | 1.0 | 1.4 | 1.2 | 1.3 | 1.3 |
| Hemoglobin (mg/dL) | 9.4 | 9.6 | 9.6 | 9.8 | 10.2 | 11.0 | 10.2 |
| Creatinine (mg/dL) | 9.78 | – | 8.60 | – | 9.47 | 10.05 | 9.28 |
| CRP (mg/L) | 5.53 | 179.72 | 213.40 | 8.53 | – | 1.99 | – |
CRP: C-reactive protein; WBC: white blood cell.
Peritonitis is the most common complication in patients on PD with a 5% mortality rate after an acute episode of peritonitis. Therefore, prevention, and an appropriate and immediate approach are essential for the long-term success. Most peritonitis cases are secondary to bacterial agents, predominantly gram-positive organisms.1
Salmonellae are gram-negative, facultative anaerobes, from the Enterobacteriaceae family. The serotypes S. Typhi and S. paratyphi are capable of causing typhoid fever and paratyphoid fever, respectively. The remaining serotypes can colonize the gastrointestinal tract, cause gastroenteritis and/or can be associated with localized infection and bacteremia.4 The eradication of Salmonella is challenging, since they can produce biofilm-associated infections and thus represent a large medical problem.3
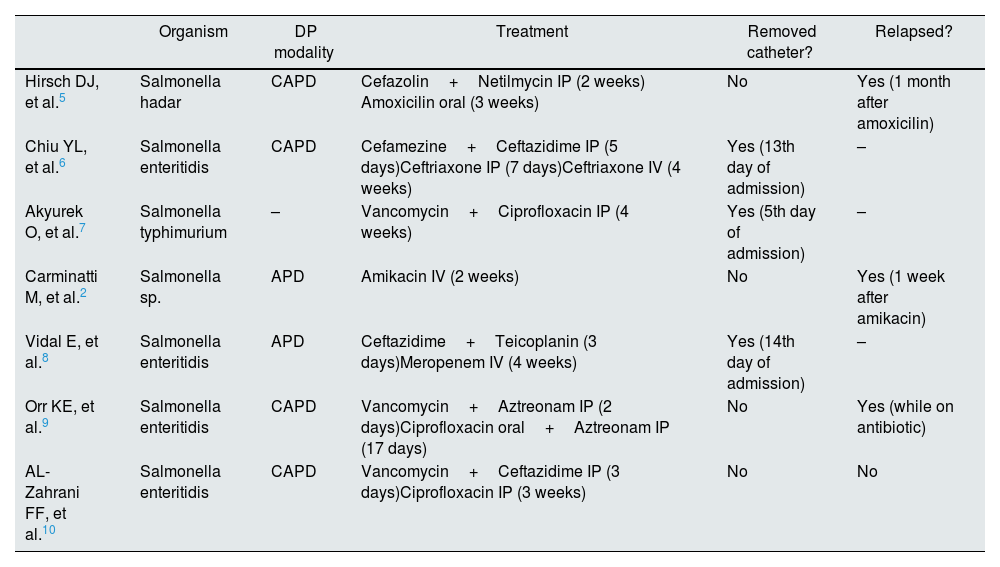
Among PD patients, Salmonella peritonitis is extremely rare. After reviewing the literature, we found seven other reports of PD-related peritonitis caused by Salmonella.2,5–10 In every case, patients were administered prolonged antibiotic treatment with or without catheter removal (Table 2). In most cases, where it was decided not to remove the catheter, peritonitis eventually relapsed. In one case, however, where the catheter was not removed, the patient completed three weeks of intraperitoneal ciprofloxacin, but no follow-up was provided. Our case is the first to provide long term follow-up after the decision not to exchange the catheter with no evidence of recurrence. However, we would like to highlight that due to the small number of described cases, there is no consensus regarding Tenckhoff catheter removal and duration of antibiotherapy. Therefore, decision making should be performed in an individualized manner.
Treatment modality, antibioterapy duration, catheter outcome of the seven reported cases of Salmonella Peritonitis in PD patients.
| Organism | DP modality | Treatment | Removed catheter? | Relapsed? | |
|---|---|---|---|---|---|
| Hirsch DJ, et al.5 | Salmonella hadar | CAPD | Cefazolin+Netilmycin IP (2 weeks) Amoxicilin oral (3 weeks) | No | Yes (1 month after amoxicilin) |
| Chiu YL, et al.6 | Salmonella enteritidis | CAPD | Cefamezine+Ceftazidime IP (5 days)Ceftriaxone IP (7 days)Ceftriaxone IV (4 weeks) | Yes (13th day of admission) | – |
| Akyurek O, et al.7 | Salmonella typhimurium | – | Vancomycin+Ciprofloxacin IP (4 weeks) | Yes (5th day of admission) | – |
| Carminatti M, et al.2 | Salmonella sp. | APD | Amikacin IV (2 weeks) | No | Yes (1 week after amikacin) |
| Vidal E, et al.8 | Salmonella enteritidis | APD | Ceftazidime+Teicoplanin (3 days)Meropenem IV (4 weeks) | Yes (14th day of admission) | – |
| Orr KE, et al.9 | Salmonella enteritidis | CAPD | Vancomycin+Aztreonam IP (2 days)Ciprofloxacin oral+Aztreonam IP (17 days) | No | Yes (while on antibiotic) |
| AL-Zahrani FF, et al.10 | Salmonella enteritidis | CAPD | Vancomycin+Ceftazidime IP (3 days)Ciprofloxacin IP (3 weeks) | No | No |
CAPD: continuous ambulatory peritoneal dialysis; APD: ambulatory peritoneal dialysis; IP: intraperitoneal; IV: intravenous.
In conclusion, we have reported the first case of Salmonella peritonitis in a peritoneal dialysis patient where catheter was not removed and provide a long follow-up without evidence of recurrence.
Authors’ contributionsJoão Carvão and José Durães provided medical care of the patient. João Carvão wrote the manuscript. Luís Resende and José Durães reviewed the literature. All authors edited the manuscript. João Carvão is the article guarantor.
Statement of ethicsInformed consent was obtained for this report.
Funding sourcesNo subsidies or grants contributed to this work.
Conflicts of interestThe authors have no conflicts of interest to declare.