Kidney is a vital organ which plays an important and irreplaceable role in detoxification and removal of xenobiotics. And therefore is vulnerable to develop various forms of injuries. Hence, making it immensely important to search for natural reno-protective compounds.
ObjectivesThis study therefore, aims to evaluate the reno-protective properties of propolis against gentamicin induced renal toxicity in mice.
MethodsThree groups of 10 male mice each were used for this study. First group served as control, the second group (Gm group) was administered orally 80mg/kg body weight gentamicin for 7 days, and the third group (GmP group) was administered same dose of gentamicin with propolis (500mg/kg body weight) for 7 days. Various parameters were used to study the renal toxicity.
ResultsGentamicin caused significant renal damage as evident by the rise in BUN levels, diminished glomeruli hypocellularity, moderately dilated tubules, and mild loss of brush border, severe infiltration, extensive tubular degeneration and presence of tubular cast. Histochemistry results show presence of collagen and reticular fibres. Immunohistochemical reactions show kidney injury (Kim-1 gene-expression), oxidative stress (MDA gene-expression), and an increase in apoptosis (caspase-3 gene-expression). Co-administration of propolis with gentamicin showed significant decrease in BUN levels, appearance of healthy glomeruli with normal cellularity, reduction of tubular injury, decrease of collagen and reticular fibres deposition, reduction of apoptosis, kidney injury and oxidative stress.
ConclusionResults presented in this study clearly show the reno-protective role of propolis against gentamicin-induced toxicity on mice kidney.
El riñón es un órgano vital que desempeña una función importante e insustituible en la desintoxicación y la eliminación de los xenobióticos y, por lo tanto, es vulnerable a desarrollar diversas formas de lesión. Esto hace muy importante la búsqueda de compuestos renoprotectores naturales.
ObjetivosEste estudio tiene como objetivo evaluar las propiedades renoprotectoras del propóleo contra la toxicidad renal inducida por gentamicina en ratones.
MétodosPara este estudio se utilizaron 3 grupos de 10 ratones macho en cada uno. El primer grupo sirvió como control, el segundo grupo (grupo Gm) recibió 80mg/kg de peso corporal de gentamicina por vía oral durante 7 días y el tercer grupo (grupo GmP) recibió la misma dosis de gentamicina con propóleo (500mg/kg de peso corporal) durante 7 días. Se utilizaron varios parámetros para estudiar la toxicidad renal.
ResultadosLa gentamicina causó daño renal significativo, como demostró el aumento de los niveles de nitrógeno ureico en sangre, la disminución de la hipocelularidad glomerular, los túbulos moderadamente dilatados y la pérdida leve del borde en cepillo, la infiltración grave, la degeneración tubular extensa y la presencia de cilindros tubulares. Los resultados de la histoquímica muestran presencia de colágeno y fibras reticulares. Las reacciones inmunohistoquímicas muestran lesión renal (expresión del gen Kim-1), estrés oxidativo (expresión del gen MDA) y un aumento de la apoptosis (expresión del gen caspasa-3). La administración concomitante de propóleo con gentamicina mostró disminución significativa de los niveles de nitrógeno ureico en la sangre, aspecto de glomérulos sanos con celularidad normal, reducción de la lesión tubular, disminución de colágeno y deposición de fibras reticulares, reducción de la apoptosis, daño renal y estrés oxidativo.
ConclusiónLos resultados presentados en este estudio muestran claramente la función renoprotectora del propóleo contra la toxicidad inducida por gentamicina en el riñón de los ratones.
Gentamicin is commonly used aminoglycoside antibiotic for the treatment of various bacterial infections. The recommended routes of administration of gentamicin are intravenous, intramuscular, intraperitoneal or topical as it is not sufficiently absorbed by the intestinal tract.1,2 However, potential clinical use of gentamicin is limited due to gentamicin-induced toxicity, even at doses slightly higher than recommended doses. Gentamicin can cause tissue injury such as nephrotoxicity, ototoxicity3,4 and liver toxicity,5 possibly through generation of free oxygen radicals. Nephrotoxicity of gentamicin arises due to its accumulation in renal cortical tubular epithelial cells.2 Although the pathogenesis of gentamicin-induced acute kidney injury (AKI) has been the focus of a large number of studies, the underlying mechanisms are not yet fully elucidated. Recent studies suggest that gentamicin nephrotoxicity is a complex and multifaceted process in which gentamicin triggers cellular responses involving multiple pathways that culminate in renal damage and necrosis.6,7 Therefore, a number of different molecular markers are being used to assess the kidney injury including Kidney Injury Molecule-1 (KIM-1), markers for apoptosis and oxidative stress.8–10
Several agents and strategies have been attempted to ameliorate gentamicin nephrotoxicity11–13 with main focus on the use of various antioxidant agents including the extracts from medicinal plants with antioxidant properties.11 However, none of these have been found safe/suitable for clinical practice due to known and unexplored side effects. Propolis a gum like substance gathered by bees from various plants and varies in colour from light yellow to dark brown,14 possesses a broad spectrum of biological activities such as anti-hepatitis and anti-arthritis, and is also known to enhance immune system.15–17 This biological activity may be attributed to its constituents obtained from plants, mainly phenolic compounds such as flavonoids. Flavonoids are well-known antioxidant possessing free radical scavenging and metal chelating activity.18 At least 38 different flavonoids have been reported in propolis.19 Some components of the propolis are absorbed and circulate in the blood and behave as hydrophilic antioxidant and save vitamin C.20 The present study therefore evaluates the potential of propolis when administered orally to protect the kidney against the harmful effects and acute nephrotoxicity of gentamicin in swiss albino mice.
Materials and methodsAnimalsSwiss albino male mice weighing 25±g were used for the experiment. These animals were acclimated to 22±1°C and were maintained under 12-h periods of light and dark each, with free access to clean water and commercial mice food. The animals were housed in polypropylene cages inside a well-ventilated room.
Experimental designMice were randomly distributed into three groups, each containing 10 mice. Group 1 mice received saline and served as control group while group 2 mice received intraperitoneal injection of gentamicin at dose of 80mg/kg for 7 consecutive days and this group was marked as Gm group. Mice in group 3 were treated as group 2 and were additionally co-administered with 500mg/kg of propolis one hour-post gentamicin injection and this group was marked as GmP group.
Kidney indexFollowing treatments as described above, each mouse was weighed; kidneys were removed and weighed. Finally, the kidney index was calculated by dividing the left kidney weight by the body weight and then multiplying by 100 and the results were statistically analyzed by SPSS software (SPSS Inc.).
Biochemical analysisBlood samples for the measurement of blood chemistry were drawn into prechilled tubes containing EDTA, and immediately placed on ice. Serum in the samples was separated by centrifugation at 3000rpm and stored at −80°C until assay. Serums were used for the estimation of blood urea nitrogen (BUN) and creatinine.
Histopathological analysisHistopathological preparationKidneys were collected and cut into small pieces, fixed in 10% neutral buffered formalin. Following fixation, specimens were dehydrated, embedded in wax, and then sectioned to 5μm thicknesses. Sections were stained with haematoxylin and eosin, Masson's Trichrome stain and Gomori silver technique. Digital images of kidneys tissues were obtained using a light microscope at a magnification of 400×.
Gene-expression localization studiesParaffin embedded kidney sections were deparaffinized in xylene and rehydrated in descending grades of alcohol and finally distilled water. Sections were then heated in citrate buffer (pH 6) in microwave for 5min, washed with PBS buffer for 5min and were incubated in peroxidase blocking solution for 10min. After blocking sections were incubated overnight at 4°C with diluted primary antibody (anti-caspase3 ab13585, anti-Kim-1, rabbit polyclonal antibody ab78494, anti-malondialdehyde ab194225). Sections were then incubated with biotinylated goat anti-mouse secondary antibody (ab128976) for 30min, followed by incubation in avidin-biotin complex for 30min. Finally DAB (ab64238) was used as chromogenic substrate for the detection of Ab binding. Stained sections were counter stained with Mayer's haematoxylin, and dehydrated within ascending grades of alcohol and cleared with two changes of xylene, mounted with cover slip based on DPX mountant, (all reagents from Abcam). Kidney sections were examined under microscope for brown immunoreactivity colour and photos at 400× magnification.
Renal pathology analysisFormalin-fixed kidney sections (5μm) were stained with haematoxylin and eosin to distinguish cell nuclei and digital images of glomeruli were recorded at 400× magnification using a light microscope. Glomerular tuft areas were measured by microscopy computer system (Motic-2000), while, glomerular cellularity was determined by counting the number of nuclei in 20 hilar glomerular tuft cross-sections per animal.
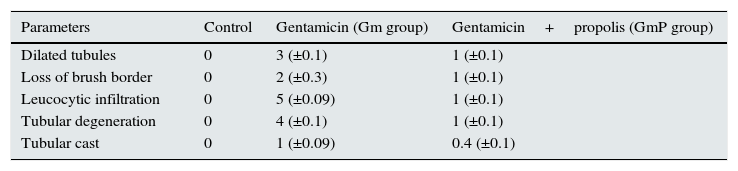
Pathological score for tubular injuryFor determining pathological score haematoxylin eosin stained preparations were evaluated under light microscope. Dilated tubules, loss of brush border, tubular casts, leukocytic infiltration and tubular degeneration in the cortical area were scored as described by Biswas et al.21 The scoring system used is described as follows. Kidneys showing no tubular injury were marked 0. While, kidneys exhibiting mild tubular injury ≤10% were given a score of 1. Similarly, kidneys showing mild (10–25%), moderate (26–50%), extensive (=51–75%) and severe (≥75%) tubular injuries were assigned a score of 2, 3, 4 and 5, respectively. Tubular cast scored as 0=negative and 1=positive.
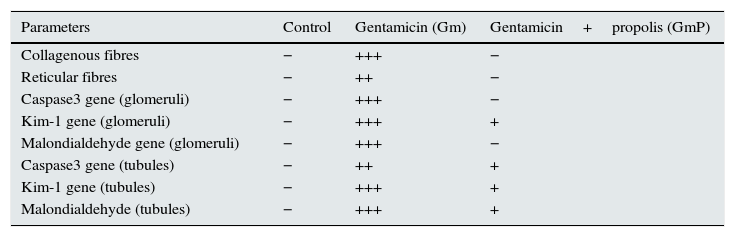
Histochemical and immunohistochemical analysisKidney sections stained with Mason's Trichrome, Gomori silver technique, Caspase 3 in glomeruli and tubules, Kim-1 in glomeruli and tubules, and malondialdehyde gene-expressions by ABC method were quantitatively scored as −=none, +=little, ++=mild and +++=intense.
Statistical analysisStatistical evaluation was carried out by using one-way ANOVA test and SPSS (16.0 software), all values were expressed as mean±SD. Values of p<0.05 were accepted as significant.
ResultsKidney index and biochemical analysisKidney index showed insignificant difference between control and gentamicin experimental groups (Table 1). Blood urea nitrogen (BUN) levels increased significantly (p<0.05) in gentamicin (Gm) and gentamicin with propolis (GmP) groups compared to control group. The Kidney index for Control, Gm and GmP group was 22, 41 and 38, respectively (Table 1). It is important to note that there was an insignificant decrease of Kidney index in GmP group (38) compared to Gm group (41). Creatinine levels showed insignificant difference in gentamicin experimental groups compared to control group (Table 1).
Change in kidney index, BUN blood serum and creatinine in blood serum following, treatment with gentamicin alone and along with propolis, in mice.
| Parameters | Control | Gentamicin (Gm group) | Gentamicin+propolis (GmP group) |
|---|---|---|---|
| Kidney index | 0.60 (±0.03) | 0.62 (±0.08), +3.3%† | 0.65 (±0.07), +8.3% |
| BUN (mg/dl) | 22.6 (±0.6) | 41 (±2.0*), +81.4% | 38 (±3.0*), +68.1% |
| Creatinine (mg/dl) | 0.4 (±0.03) | 0.36 (±0.05), −10% | 0.33 (±0.02), −17.5% |
Values presented in parenthesis as mean±SD (standard deviation).
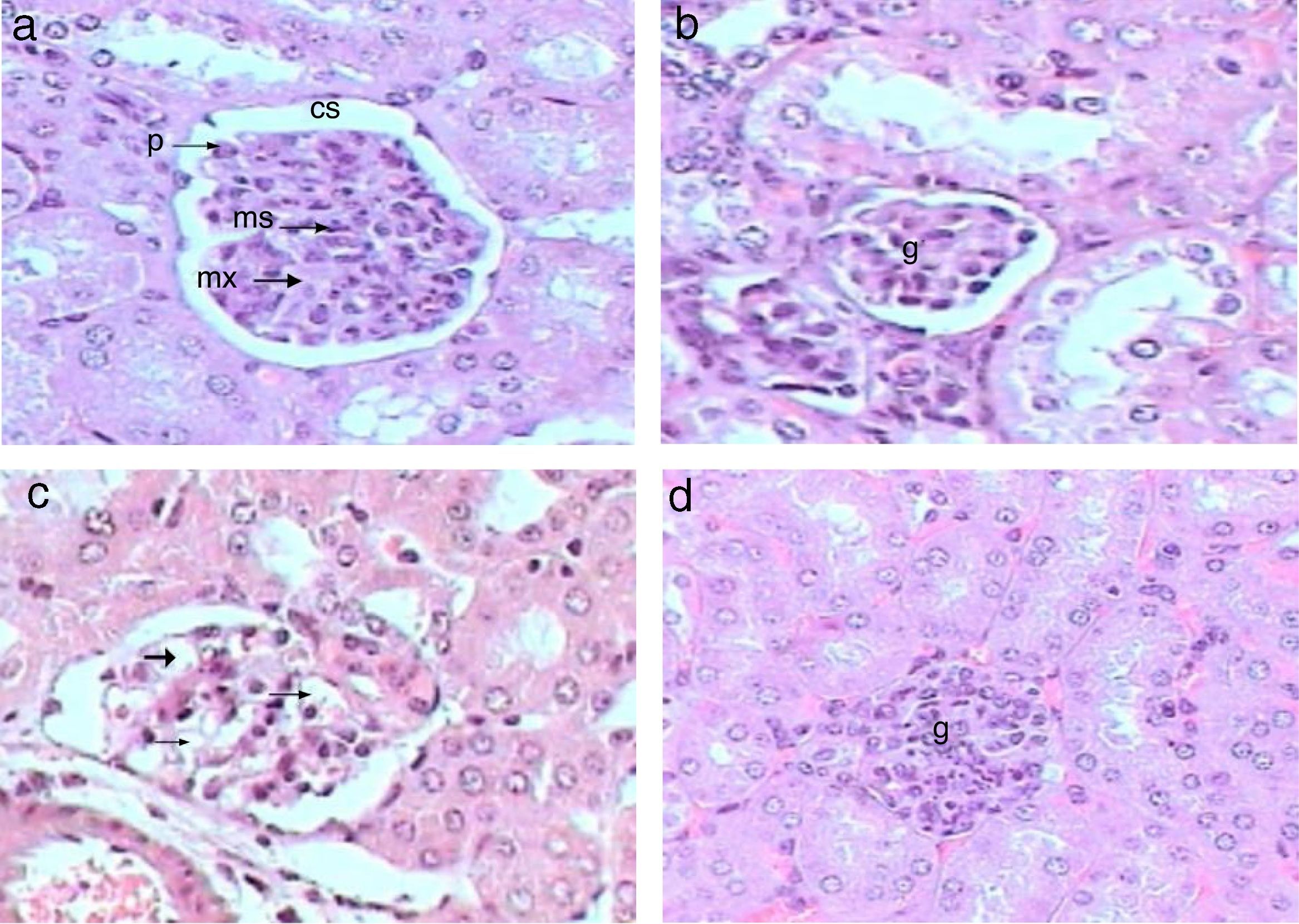
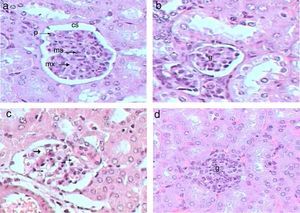
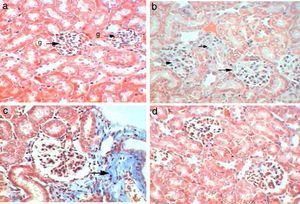
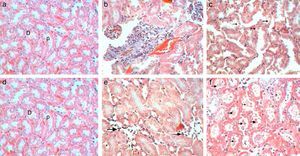
Control kidney exhibit normal glomeruli score (4.3μm3), glomerular area, and (30C/gcs) cells (Table 2) with abundant podocytes, mesangial cells with healthy mesangial matrix in between and normal capsular space (Fig. 1a). Kidney sections of Gm mice group showed diminished glomeruli that scored significant decrease in area (2.4μm3) and cellularity (20C/gcs) compared to control group p<0.05, in addition to severe degeneration in mesangial matrix (Fig. 1b and c). Whereas, GmP mice revealed relatively healthy glomeruli evident from large podocytes, abundant mesangial cells and healthy mesangial matrix (Fig. 1d), scoring 3.8μm3 glomerular area and (27C/gcs) glomerular cells with insignificant difference compared to control group and significant increase compared to gentamicin group (Table 2).
Glomerular areas and glomerular cellularity of Control, Gm and GmP mice groups.
| Parameters | Control | Gentamicin (Gm group) | Gentamicin+propolis (GmP group) |
|---|---|---|---|
| Glomerular area (μm3) | 4.3 (±2.8) | 2.4 (±1.5*), −44%† | 3.8 (±2), −11% |
| Glomerular cellularity (cells/gcs) | 30 (±1.2) | 20 (±0.9*), −33% | 27 (±1.2), −10% |
Values in parenthesis are mean±SD (standard deviation).
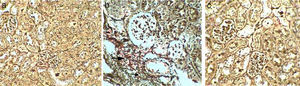
Control kidney sections stained with Masson's Trichrome showed abundant glomerular cells without any depositions of collagenous fibres inside glomeruli or in between cortical tubules (- to collagenous fibres) (Table 4, Fig. 2a). Whereas, kidney sections of Gm mice showed intense depositions of collagenous fibres and stained blue by Masson's Trichrome in the glomeruli and also in between cortical tubules (+++) (Table 4, Fig. 2b and c). Kidney sections of GmP mice show no collagenous fibres depositions in glomeruli or in between tubules (−) (Table 4, Fig. 2d).
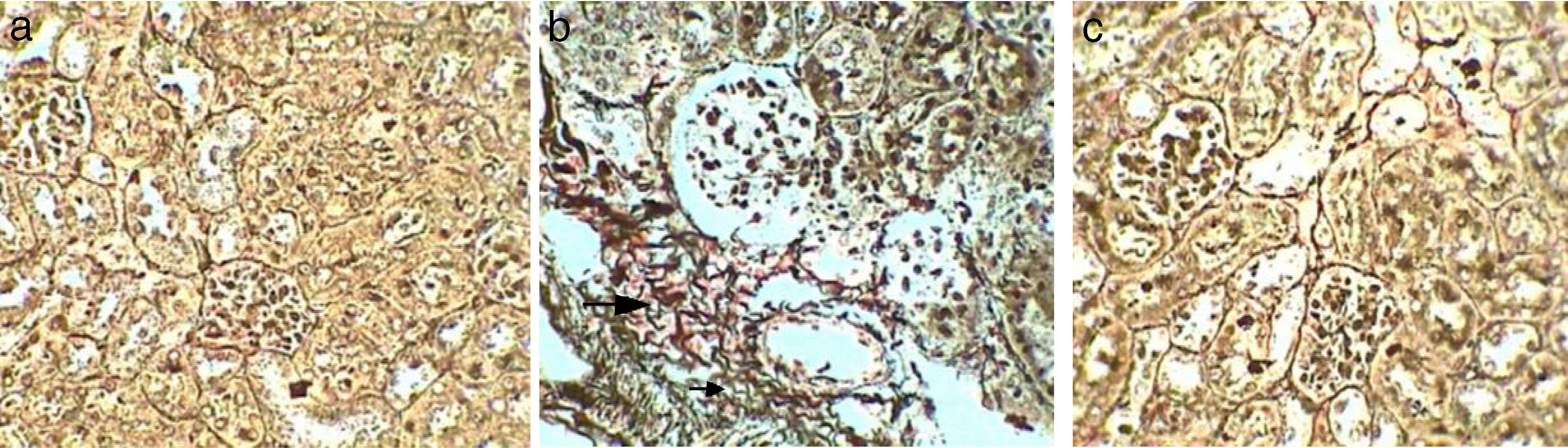
Control kidney sections stained with Gomori silver technique showed no deposition of reticular fibres (−) (Fig. 3a). Whereas, kidney sections of Gm showed mild depositions of brown reticular fibres (++) in necrotic areas (Fig. 3b). While, kidney sections of GmP mice show no reticular fibres depositions (−) (Table 4, Fig. 3c).
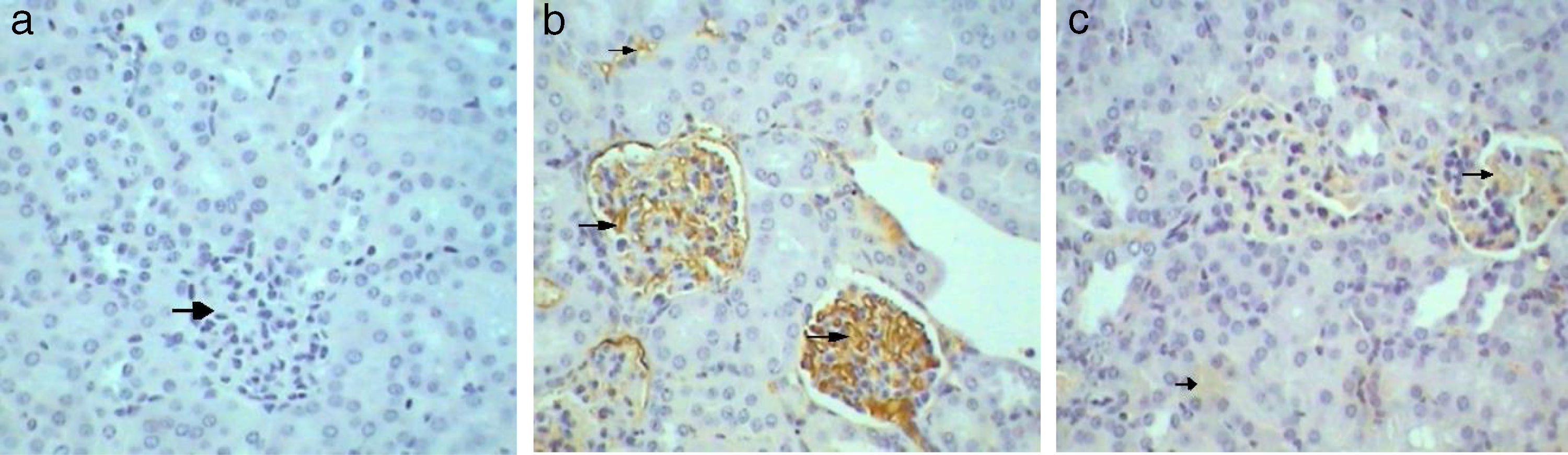
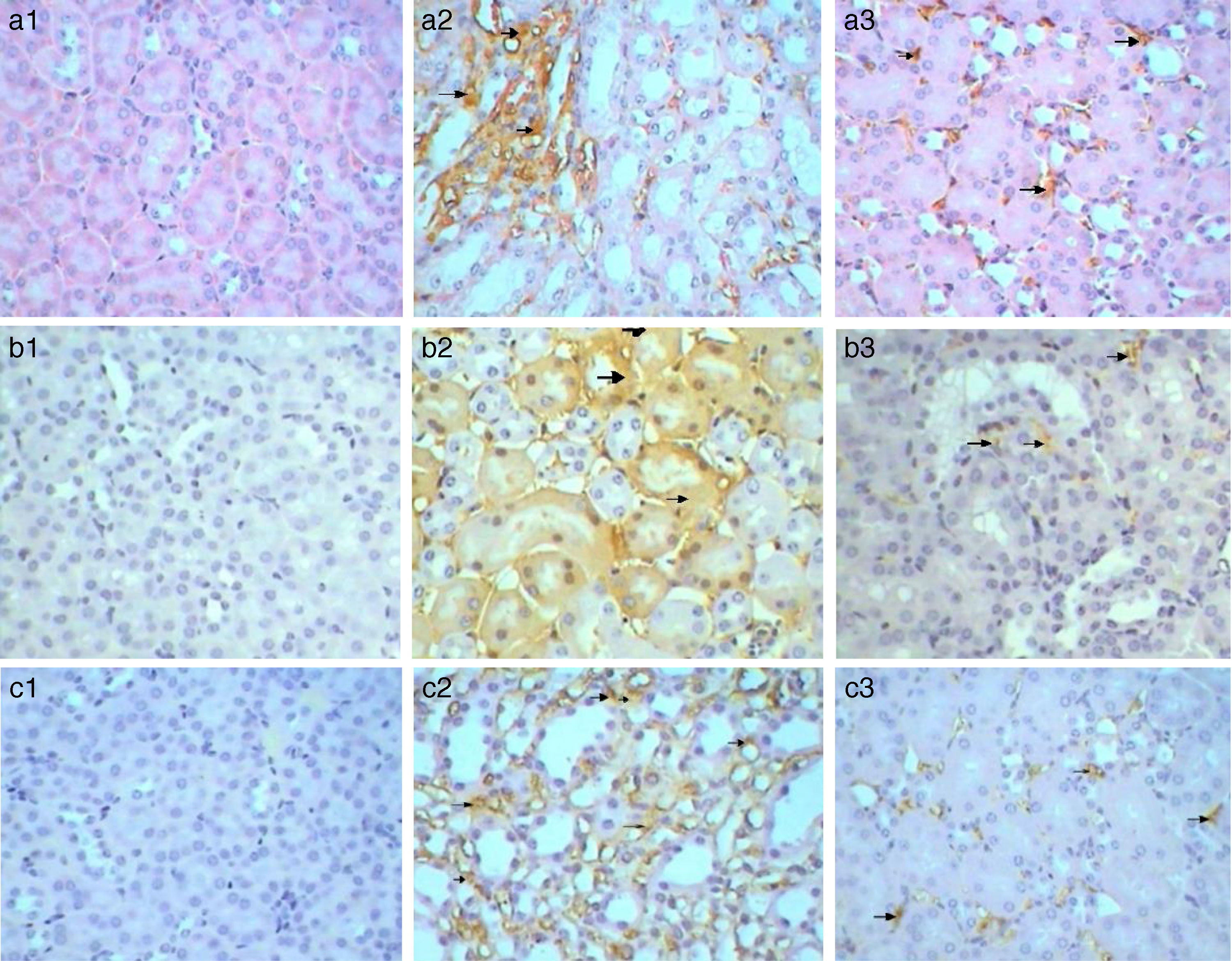
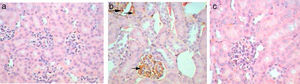
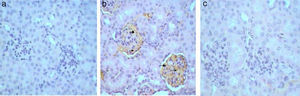
Kidney sections stained by Avidin Biotin Complex (ABC) immunohistochemistry method for caspase-3 gene-expression show no immunoreactivity (−) in the kidney sections of control mice group (Fig. 4a) and in kidney sections from GmP mice (Fig. 4c). Whereas, kidney of Gm show intense brown immunoprecipitation (+++) inside the glomeruli (Fig. 4b, Table 4), indicating apoptosis.
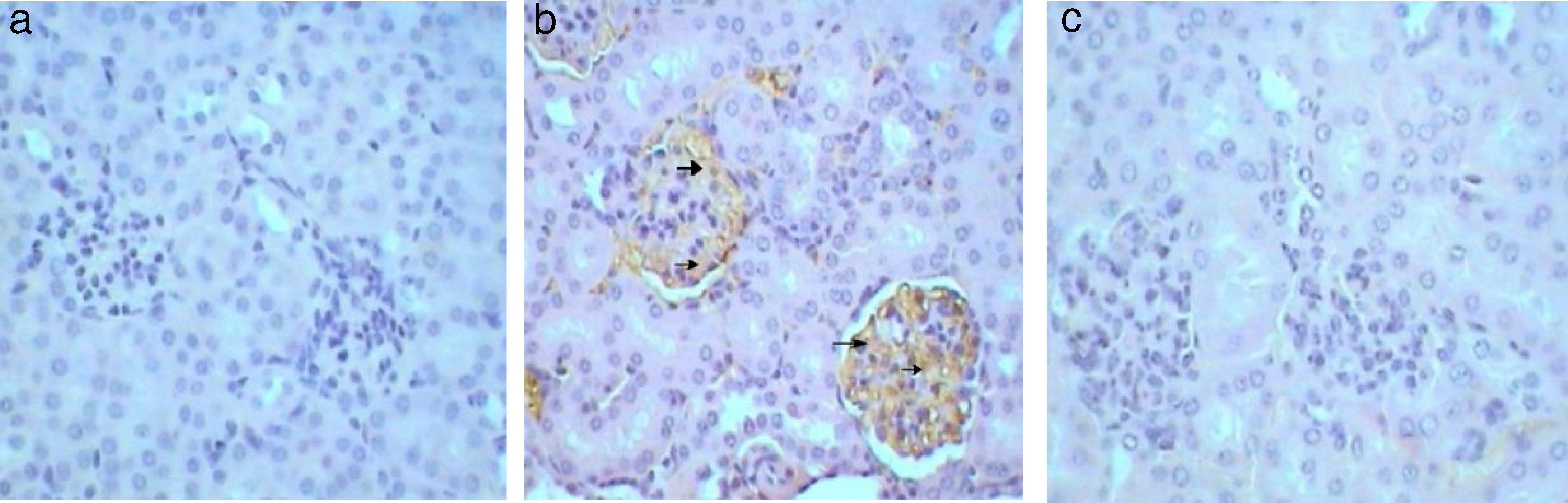
Similarly, Kim-1 gene-expression shows no immunoreactivity (−) in control sections (Fig. 5a). Whereas, an intense immunoprecipitation was observed in glomeruli and cortical tubules in sections of Gm mice kidney (+++) (Fig. 5b). A slight ameliorative effect of propolis was evident from weak brownish immunoprecipitation observed in sections of GmP mice kidney (+) (Table 4, Fig. 5c). Kidney sections stained for Malondialdehyde (oxidative stress Marker) show no immunoprecipitation (−ve) in untreated control sections (Fig. 6a) almost similar immunoreaction was observed in GmP mice (Fig. 6c). Whereas, intense immunoprecipitation was observed in glomeruli sections of Kidney from Gm mice group (+++) (Fig. 6b, Table 4).
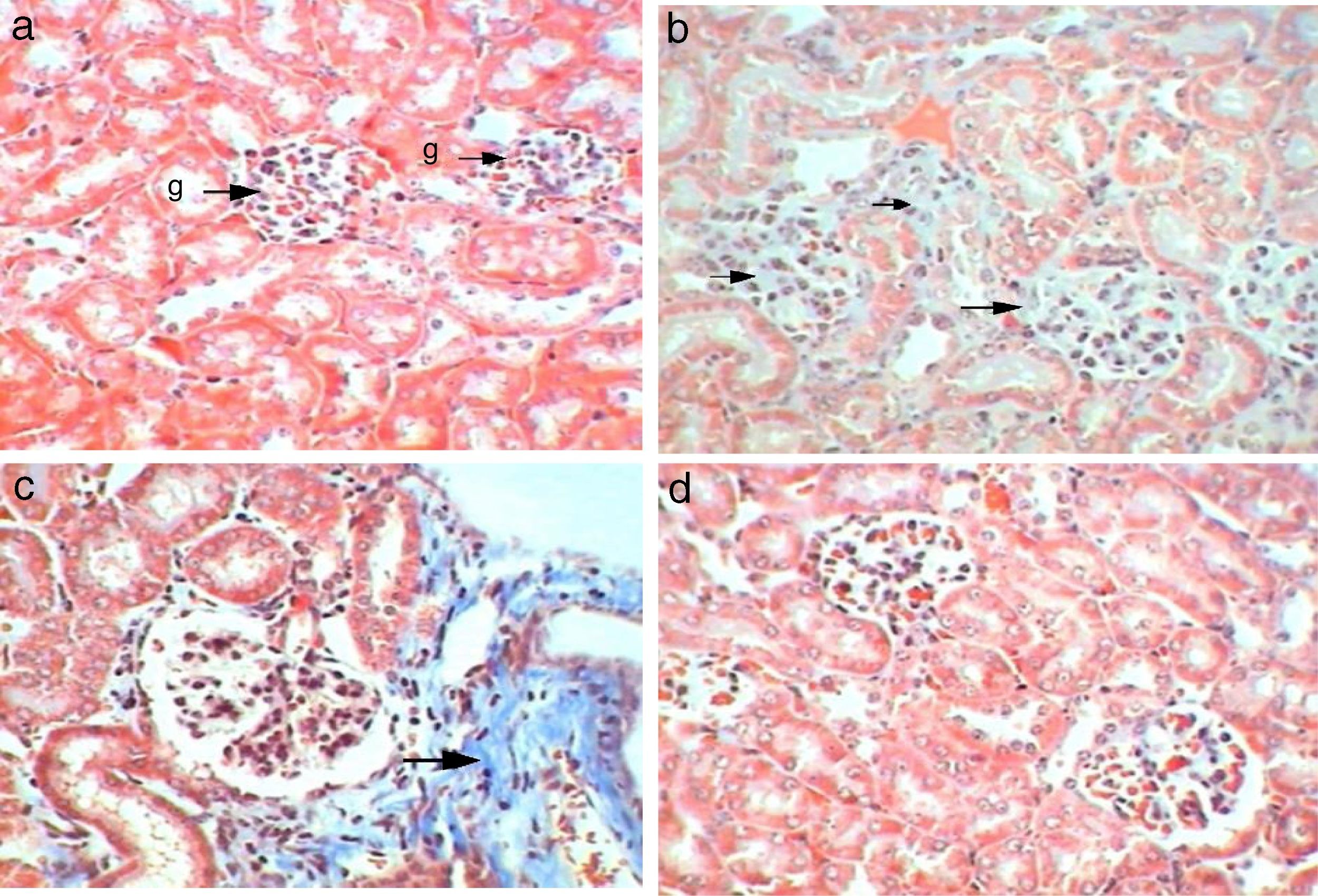
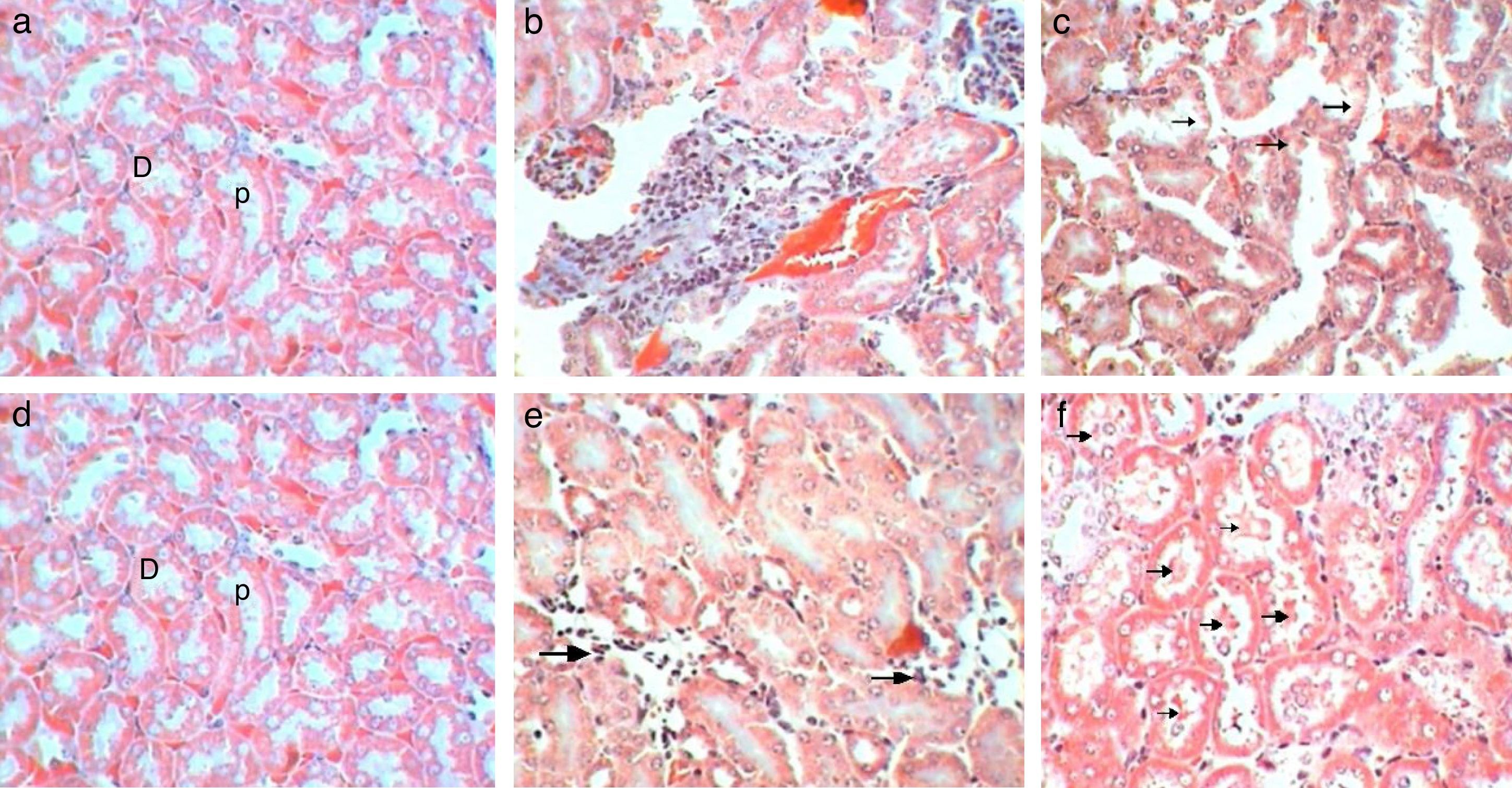
Tubular analysisControl kidney sections showed normal tubules without dilatation and proximal tubules appeared filled because of the long microvilli of the brush border and aggregates of small plasma proteins bound to this structure, by contrast lumens of distal tubules appeared empty (Fig. 7a). Sections of Gm mice kidney showed mild dilatation with a pathological score of 2 with empty lumens of proximal tubules score (3), moderate loss of pathological score (Fig. 7b). Whereas, sections of GmP mice scored 1, with mild injuries, dilatation and loss of brush borders (Table 3, Fig. 7c).
Pathological score of tubular injury in control, Gm and GmP experimental group of mice.
| Parameters | Control | Gentamicin (Gm group) | Gentamicin+propolis (GmP group) |
|---|---|---|---|
| Dilated tubules | 0 | 3 (±0.1) | 1 (±0.1) |
| Loss of brush border | 0 | 2 (±0.3) | 1 (±0.1) |
| Leucocytic infiltration | 0 | 5 (±0.09) | 1 (±0.1) |
| Tubular degeneration | 0 | 4 (±0.1) | 1 (±0.1) |
| Tubular cast | 0 | 1 (±0.09) | 0.4 (±0.1) |
The data presented in parenthesis are ±SD (standard deviation).
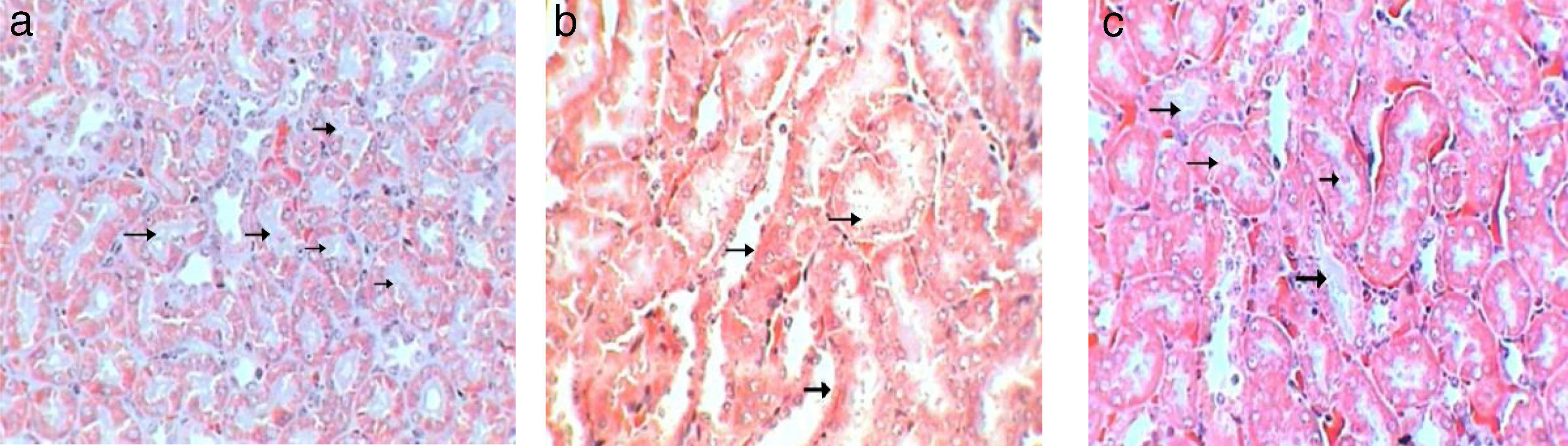
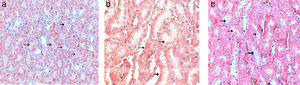
Control sections show (score 0) no leucocytic infiltration, tubular degeneration and tubular cast (Fig. 8a). While sections of Gm mice kidney show severe leucocytic infiltration (score 5, Fig. 8b), extensive tubular degeneration (score 4, Fig. 8c) and presence of tubular cast (Score 1, Fig. 8d, Table 3). Whereas, sections of GmP scored show mild leucocytic infiltration (Score 1), and tubular degeneration but do not show tubular cast (score 0, Fig. 8e).
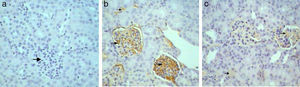
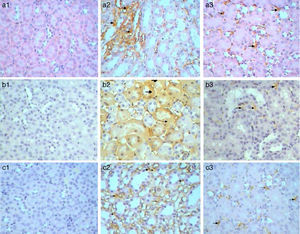
Immunohistochemical analysis of control mice shows no immunoreactivity in control sections (−) for caspase 3 (Fig. 9a1). Whereas, mild (++) immunoprecipitates were seen in tubules kidney of Gm mice (Fig. 9a2). In GmP mice group however, there was a significant decrease in the intensity of immunoprecipitation (+) (Table 4, Fig. 9a3).
Histochemical and immunohistochemical analysis in control, gentamicin (Gm), gentamicin treated with propolis (GmP) groups: −, means negative; +, little; ++, mild; +++, extensive.
| Parameters | Control | Gentamicin (Gm) | Gentamicin+propolis (GmP) |
|---|---|---|---|
| Collagenous fibres | − | +++ | − |
| Reticular fibres | − | ++ | − |
| Caspase3 gene (glomeruli) | − | +++ | − |
| Kim-1 gene (glomeruli) | − | +++ | + |
| Malondialdehyde gene (glomeruli) | − | +++ | − |
| Caspase3 gene (tubules) | − | ++ | + |
| Kim-1 gene (tubules) | − | +++ | + |
| Malondialdehyde (tubules) | − | +++ | + |
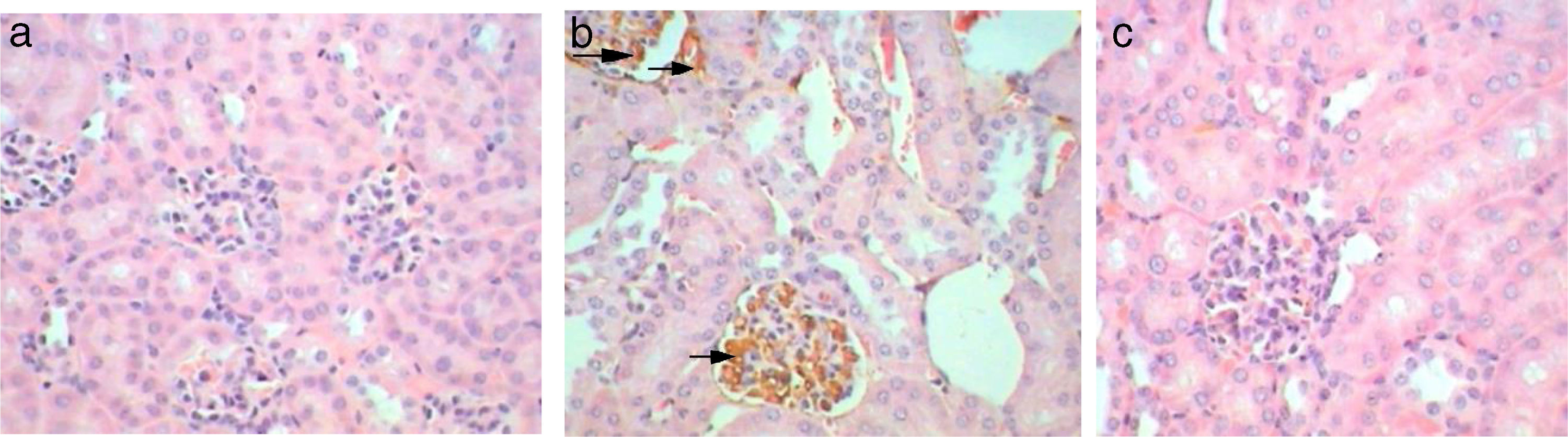
Kim-1 gene-expression also shows no immunoreactivity in control sections (−) (Fig. 9b1). Whereas, intense (+++) immunoprecipitation was observed in tubules of Gm mice kidney (Fig. 9b2). Moreover, in GmP mice (little, + Table 4) the intensity of Kim-1 gene immunoprecipitation was very low (Fig. 9b3). Kidney sections stained for Malondialdehyde (oxidative stress Marker) gene-expression showed no immunoreactivity in control sections (−) (Fig. 9c1). Whereas, intense (+++) brownish immunoprecipitates were seen in tubules of Gm mice kidney (Fig. 9c2), and very low intensity of (+, Table 4) immunoprecipitates was found in the tubules of Gmp mice kidney (Fig. 9c3).
DiscussionResults presented in this study confirmed that gentamicin administration caused marked changes in kidney tubules may be due to gentamicin reabsorption in proximal convoluted tubules, causing degeneration and necrosis of the epithelial cells of the tubules. These changes are manifested by dilated tubules, loss of brush border, severe leucocytic infiltrations, tubular degeneration and presence of tubular casts. These findings are in agreement with previous studies.22-24 Co-administration of propolis with gentamicin revealed significant improvement in kidney tubules marked by the absence of tubular casts, reduction of infiltration, degeneration and tubular dilatation. Azab et al.25 also reported similar effect of propolis, wherein co-administration of propolis with gentamicin, resulted in normal epithelial lining with brush borders in proximal convoluted tubules. However, some tubules appeared regenerating with disrupted brush borders.
Han et al.26 has shown the activation of proapoptotic proteins in kidneys exhibiting nephrotoxicity. Caspases often used as a marker to study apoptosis, are form the family of endoproteases that provide critical links in cell regulatory networks controlling inflammation and cell death.27 Sahu et al.28 has shown that Gentamicin results in apoptosis in glomeruli and tubules. While, this toxicity was ameliorated by the co-administration of propolis. Renoprotective effect of Brazilian red propolis has also been demonstrated by Teles et al.29 Other biomarkers to study nephrotoxicity include Kidney injury molecule 1. Prozialeck et al.30 has suggested the use of KIM-1 as a nephrotoxicity biomarker in preclinical studies of drug candidates. Furthermore, Food and Drug Administration (USA) has also recently recognized KIM-1 as an appropriate biomarker for renal injury in preclinical studies of pharmacological agents. Besides being a sensitive diagnostic marker of nephrotoxicity, KIM-1 also has predictive value for AKI in patients undergoing cardiac surgery.31 Results obtained in our study confirmed that gentamicin administration produced severe kidney injury as evident from intense immunoreactions of kim-1 gene in glomeruli and tubules. These findings are in agreement with the reports of Chen et al.,32 Mcduffie et al., 33 and Qiu et al.34 As in these studies also an intense immunoreaction of Kim-1 was observed following exposure to gentamicin. Interestingly, a decrease in kim-1 immunoreaction was observed in this study when Gentamicin was co-administered with propolis; a trend which was also observed in caspase-3 immunoreactions.
Another mode through which gentamicin exert its nephrotoxicity, is through the generation of Reactive oxygen species (ROS) or oxidative stress.35 These ROS target a number of biomolecules including lipids. Malondialdehyde (MDA) is the principal and most studied product of polyunsaturated fatty acid peroxidation. And hence is considered as an important marker of lipid peroxidation.36 In agreement with previous studies,37 gentamicin administration produced intense immunoreaction of (MDA) gene as an oxidative stress marker in glomeruli and tubules confirming the gentamicin mediated oxidative stress in kidney tissue. However, oral administration of propolis resulted in a decrease of MDA immunoprecipitation suggesting a decrease in oxidative stress. However, the pathway through which propolis result in this change is not known.
Based on the results presented in this study, it can be concluded that propolis is a good renoprotective agent and can effectively ameliorate the renotoxicity of gentamicin.
Conflicts of interestThe authors declare no conflicts of interest.
Thanks and sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No. (RG-1435-030) and its perfect support for this project.