Kidney transplantation is the optimal therapy for end-stage kidney disease but limited by the available number of organs. Using HCV+ donors, both in HCV+ and HCV− recipients, is a rational response to the organ shortage. We review the historic experience using HCV+ donors in HCV+ recipients and assess long-term results. We also discuss contemporary practices, including the transplantation of HCV-viremic kidneys into HCV− recipients with different approaches to posttransplant HCV therapy.
El trasplante renal es el tratamiento óptimo de la insuficiencia renal terminal pero está limitado por el número de órganos disponibles. El uso de los riñones de los donantes VHC+, tanto en receptores VHC+ como VHC−, es una respuesta racional a la escasez de órganos. En este artículo revisamos la experiencia histórica usando riñones de donantes VHC+ en receptores VHC+ y evaluamos los resultados a largo plazo. Además, discutiremos las prácticas contemporáneas incluyendo el trasplante de órganos VHC+ virémicos en receptores VHC− con diferentes opciones de tratamiento VHC postrasplante.
Kidney transplantation is the treatment of choice for patients with end-stage renal disease.1 One of the challenges in transplantation today is that the number of patients on the waiting list worldwide has been increasing at a faster rate than the number of donors and organs available for transplantation.2 To mitigate this organ shortage, the use of expanded criteria donors and donors with transmissible diseases such as hepatitis C (HCV) has emerged over the last thirty years.3,4 Since 1990 kidneys from hepatitis C (antibody) positive donors have been transplanted safely into HCV antibody positive recipients 5 and this option has been recommended by European and KDIGO guidelines.6,7
Interferon-free direct acting antiviral agents (DAAs) have been available since late 2013 and have been shown to be highly effective for the treatment of HCV without an increased risk of rejection.8 This important advance has facilitated the use of HCV positive donors in kidney transplantation and importantly, created the possibility to transplant these kidneys into HCV negative recipients.9
Herein we provide a review of the use of HCV positive donors in renal transplantation considering two eras: first in the period before DAA treatment and second after DAAs became available. We also provide an approach to the treatment of HCV in kidney transplant recipients, recognizing that this remains a rapidly evolving field.
Transmission of HCV by kidney transplantsPereira et al. first described in 1989 that HCV infection was transmitted to organ transplantation.10 This important finding was replicated and led to the routine screening of potential donors for evidence of HCV infection. Notably, the probability of disease transmission through kidney transplantation ranged from 14% to 100%, depending on the cases series.11–15 Furthermore, in the experience from Boston, 50% of the patients infected through kidney transplantation developed chronic liver disease something otherwise infrequent in other series.13,14 Differences concerning the viral load in the transplanted organ, the infectivity of the HCV strain, the volume of the preservation solution, the preservation method used, and the diagnostic tests applied explain these heterogeneous results.13
As the transmission of HCV infection to organ transplantation was unquestionable and therefore there was soon a general consensus that kidneys from HCV+ donors (HCVD+), irrespective of HCV RNA, should not be transplanted into recipients with a negative HCV serology (HCVR Ab−). In fact several countries in the world, for instance in Europe, clearly prohibited this option.6
In parallel the question remained whether these organs could be safely transplanted into HCV antibody positive recipients (HCVR Ab+). Anti-HCV antibodies do not confer immunity and do not necessarily indicate an active viremic state. Therefore, this approach would not completely avoid disease transmission. Additionally, superinfection with a different HCV genotype could potentially occur.16 However, universally discarding kidneys from HCVD+ was also considered an unacceptable policy due to ongoing organ shortages.
Use of HCV+ donors – the 1990s to 2014Experiences from single centersIn 1990 a pilot experience using kidneys from HCVD Ab+ into HCVR Ab+ was started in Spain. In the short-term the incidence of biochemical liver disease and survival figures among HCVR+ was similar regardless of the donor HCV serology.5 However, when HCV RNA was retrospectively assessed in donor and recipients via PCR, five HCV RNA negative recipients were found to have been transplanted with kidneys from donors who were HCV RNA+. Four of five recipients became HCV RNA+ after transplant and two developed biochemical liver disease. As a result, our policy was modified and accepted by the Spanish Health Authorities in 1993, limiting the use of HCVD+ kidneys to HCV RNA+ patients on the waiting list. Testing for HCV RNA in patients in the wait list every 6 months was also required.17
Other single center experiences using the same approach were published with similar short-term results.18–22 Some of these series demonstrated that time on the wait list for HCVR+ was ignificantly shorter when they received kidneys from HCVD+.19,20 It is important to remark that based in these experiences, European Best Practice Guidelines for renal transplantation6 and 2008 KDIGO HCV Guidelines7 recommended the use of kidneys from HCVD+ in HCV RNA+ recipients.
Long-term safety of this policy was provided in 2011 by the two hospitals from Spain with more than seventeen years of experience transplanting HCV+ D. In this study with a mean follow-up 74.5 months, 162 HCVR+ were transplanted with HCVD+ versus 306 HCVR+ transplanted from HCVD−. The most striking feature in this large study was that donor HCV+ serology was not identified as an independent risk factor for death, graft loss or severe liver disease in the multivariate analysis.23 This work had limitations: information of HCV RNA among HCVD+ was lacking, genotypes of both donors and recipients were absent and liver histology was not systematically assessed. In spite of these limitations this was the largest published experience with a detailed long-term assessment of the safety of this option for transplantation with HCVD+.
The experience from the University of Wisconsin showed that transplanting HCVD+ kidneys as opposed to HCVD− into HCVR+ provided similar graft survival but compromised patient survival in the long-term. The high incidence of new onset diabetes after transplantation (NODAT) among HCVR+ could explain this decreased patient survival. In spite of this observation, it is important to remark that HCVR+ who received kidneys from HCVD+ had better survival than those who remained on the waiting list.24
Registry dataOlder studies using data from the United States Renal Data System Registry (USRDS) reported a decreased patient survival in kidney transplant recipients transplanted from HCVD+ versus HCVD− irrespective of the HCV serology of the recipient; this difference became apparent 2 years after transplantation.25–27 The high incidence of NODAT among recipients of kidneys from HCVD+ could be the underlying reason. These authors concluded that caution is necessary using HCVD+. However, these transplants were more often from older donors and performed in older age or African American recipients, which may have biased the results. Notably, there was a lack of a specific policy regarding the use of HCV+ D in the US and these kidneys were transplanted into both HCV antibody positive and negative recipients.
However, US data has been able to demonstrate potential benefits using kidneys from HCVD+. Transplantation of HCVR+ with kidneys from HCVD+ demonstrated that the time on the waitlist was 310 days less that the average waiting time for transplantation at their center and 395 days less than their counterparts at the same center who waited for HCVD− kidneys. More importantly, other large registry analysis indicated that receiving a kidney from an HCVD+ was independently associated with improved patient survival compared with remaining on the waiting list.28
A recent analysis of the United Network for Organ Sharing registry created a propensity score-matched group of HCVR+ who received kidneys from HCVD+ in comparison with those received HCVD− kidneys. The authors found that transplantation with HCVD+ was associated with an increased risk of death and graft loss compared with those received HCVD−. However, using HCVD+ with prompt initiation of DAAs, can shorten the wait for renal transplantation and maximize organ utility for all candidates on the wait list; recipients should be adequately counseled about the risks and potential benefits of HCVD+ kidneys.29
Underutilization of HCV+ donorsAlthough clinical guidelines recommended the use of HCVD+ into HCVR+ this option has remained somewhat controversial6,7 and clearly underutilized. In the US, of 93,825 deceased donors procured between 1995 and 2009, HCVD+ kidneys were 2.60-times more likely to be discarded and of 6830 HCV+ recipients transplanted during that time period, only 29% received HCVD+ kidneys.30 Thus, there is an opportunity to expand the use of HCVD+ among HCV+ recipients.31,32
Several reasons could explain this low use of HCVD+: restrictions by national health policies and/or center-specific protocols, the small but increased risk of death and graft loss among HCVR+, the absence of a safe antiviral treatment post-transplantation and the specific problems of HCVR+ on dialysis. These recipients usually have a long history of renal disease, frequent comorbidities and a high immunological risk. In that scenario is easy to conclude that there was a surplus HCV+ organs due to a lack of an appropriate recipients. To improve the utilization of HCV+ kidneys organizational measures including offering these kidneys for pre-emptive transplantation has been suggested.33
In summary, in the pre-direct acting antiviral era transplantation of kidneys from HCVD+ into HCVR+ was considered safe in the long-term showing a small risk of death, graft loss and severe liver disease. Most importantly patients who received HCVD+ have a better chance to live that those who remain on the waitlist and dialysis.
Use of HCV+ donors in the direct acting antiviral eraHCV+ donor overviewWhen evaluating the HCV+ donor it is important to distinguish between antibody and RNA status. HCV antibodies are not neutralizing and persist even in individuals who have been successfully treated or spontaneously cleared the infection; thus, a HCV antibody positive donor's capacity to transmit infection depends on their HCV RNA status.34 HCV RNA or nucleic acid amplification test (NAT) positive donors are actively viremic and capable of transmitting HCV infection.
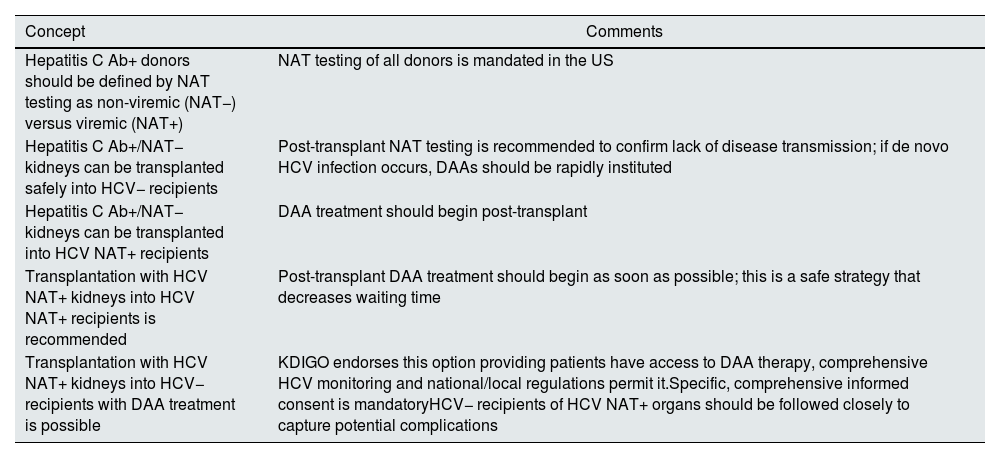
HCV Ab+/NAT− donorsThere are several reports of using HCV Ab+/NAT− donors in HCV− recipients.35,36 HCV transmission rates are essentially nil and related to false negative NAT testing. We recommend the use of HCV Ab+/NAT− kidneys in all kidney recipients, independent of recipient antibody or NAT status (see Table 1).
Summary of key concepts in the use of hepatitis C positive donors in kidney transplantation.
| Concept | Comments |
|---|---|
| Hepatitis C Ab+ donors should be defined by NAT testing as non-viremic (NAT−) versus viremic (NAT+) | NAT testing of all donors is mandated in the US |
| Hepatitis C Ab+/NAT− kidneys can be transplanted safely into HCV− recipients | Post-transplant NAT testing is recommended to confirm lack of disease transmission; if de novo HCV infection occurs, DAAs should be rapidly instituted |
| Hepatitis C Ab+/NAT− kidneys can be transplanted into HCV NAT+ recipients | DAA treatment should begin post-transplant |
| Transplantation with HCV NAT+ kidneys into HCV NAT+ recipients is recommended | Post-transplant DAA treatment should begin as soon as possible; this is a safe strategy that decreases waiting time |
| Transplantation with HCV NAT+ kidneys into HCV− recipients with DAA treatment is possible | KDIGO endorses this option providing patients have access to DAA therapy, comprehensive HCV monitoring and national/local regulations permit it.Specific, comprehensive informed consent is mandatoryHCV− recipients of HCV NAT+ organs should be followed closely to capture potential complications |
Modified from Morales and Sawinski, Clinical Transplantation 2019, 33(12):e13739.
All kidney transplant candidates are screened for HCV infection as part of the transplant evaluation. Transplant candidates who are HCV NAT+ should be encouraged to accept HCV NAT+ kidneys. HCV treatment with DAAs should be implemented as soon as reasonably possible in the post-transplant setting. Previous cost-effectiveness analyses have concluded that it is more cost effective to treat most HCV NAT+ recipients in the posttransplant setting, rather than while on dialysis37; however, these analyses did not account for the rapid increase in use of HCV NAT+ donors in NAT− recipients and the resultant increased competition (and waiting times) for HCV NAT+ organs.
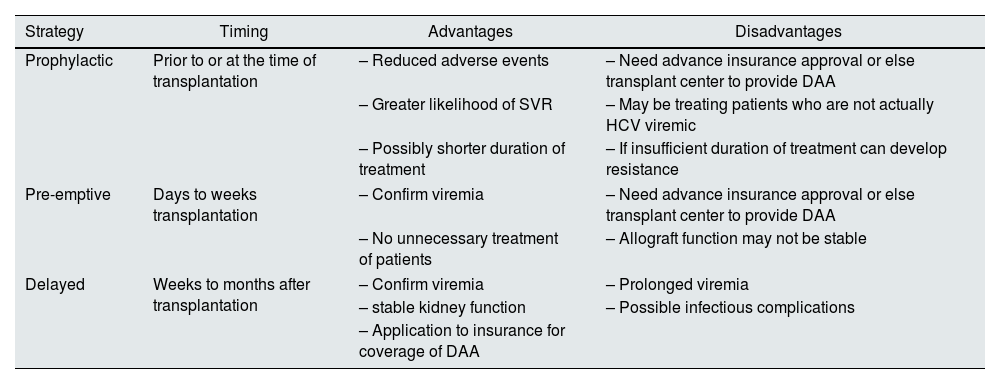
Use of HCV-infected donors in NAT− recipientsThe first interferon-free DAAs were approved by the US Food and Drug Administration in late 2013. DAAs have made the use of HCV viremic donors in HCV negative recipients with rapid post-transplant resolution of HCV infection widely possible. These transplants have been performed both in clinical trials as well as the “real-world” setting. The initiation of DAA therapy, with respect to transplantation, can be done in a prophylactic, pre-emptive (where treatment is initiated as soon as detectable viremia is documented) or delayed fashion; each approach has its own advantages and disadvantages that need to be considered (see Table 2).
DAA timing with respect to transplantation in HCV viremic donor to HCV negative recipients.
| Strategy | Timing | Advantages | Disadvantages |
|---|---|---|---|
| Prophylactic | Prior to or at the time of transplantation | – Reduced adverse events | – Need advance insurance approval or else transplant center to provide DAA |
| – Greater likelihood of SVR | – May be treating patients who are not actually HCV viremic | ||
| – Possibly shorter duration of treatment | – If insufficient duration of treatment can develop resistance | ||
| Pre-emptive | Days to weeks transplantation | – Confirm viremia | – Need advance insurance approval or else transplant center to provide DAA |
| – No unnecessary treatment of patients | – Allograft function may not be stable | ||
| Delayed | Weeks to months after transplantation | – Confirm viremia | – Prolonged viremia |
| – stable kidney function | – Possible infectious complications | ||
| – Application to insurance for coverage of DAA | |||
There are now multiple regimens available, including options with pangenotypic activity. The most current version of the American Association for the Study of Liver Diseases/Infectious Diseases Society of America guidelines38 recommend glecaprevir-pibrentasvir, ledipasvir-sofosbuvir or sofosbuvir-velpatasvir as first-line treatment for HCV in kidney transplant recipients, with elbasvir-grazoprevir as an alternative option for individuals with genotype 1 or 4 infection only. The two pangenotypic options consist of protease-inhibitor based therapy with glecaprevir-pibrentasvir or a NS5A/B-based regimen of sofosbuvir-velpatasvir; ledipasvir-sofosbuvir is not effective against genotype 2 or 3.38 The rare treatment failures that are experienced by patients with NS5A or sofosbuvir-based regimens can often be resolved with sofosbuvir-velpatasvir-voxilaprevir and achieve a 98% sustained viral response.39 While treatments targeted for specific genotypes, like sofosbuvir-ledipasvir, are still prescribed, pangenotypic regimens can be used safely for all patients, with increased prescriber comfort and their universal activity against all HCV genotypes makes them particularly well suited to algorithmic approaches.
Each regimen has its own specific advantages and safety profiles.38 While protease-inhibitor regimens are not impacted by kidney function, they have issues with drug–drug interactions. The concomitant use of cyclosporine increases serum levels of elbasvir, grazoprevir, glecaprevir and voxilaprevir; this combination is to be avoided. Glecaprevir-pibrentasvir, ledipasvir-sofosbuvir and sofosbuvir-velpatasvir-voxilaprevir can interact with statins; likewise, consideration should be given to temporarily discontinuing acid reducing agents, which can compromise DAA effectiveness. Sofosbuvir-based regimens were initially contraindicated in patients with an eGFR<30ml/m, but recent studies have demonstrated excellent safety and efficacy for sofosbuvir even in a dialysis population.40 Treatment with sofosbuvir is avoided in patients taking amiodarone, which may be necessary in patients with post-operative atrial fibrillation, due to a risk of severe bradycardia.
Clinical trialsThe first clinical trial describing the use of HCV viremic kidneys in HCV negative recipients with early post-transplant DAA treatment was published in 2017.41 Since that time there have been numerous additional trials conducted.42–46 Clinical trials have taken either the prophylactic or pre-emptive treatment approaches; DAAs have generally been supplied by the pharmaceutical manufacturer sponsoring the trial. Across trial participants, all have achieved a sustained viral response (SVR-12) without relapse and DAAs were generally well tolerated. Complications from transient HCV infection were typically minimal and most often included a temporary increase in transaminases41,43; in one study42 there was a single patient who developed focal segmental glomerular sclerosis and an increased frequency of de novo donor specific antibodies, but these observations have not been replicated in other cohorts. Outside the United States, encouraging results have been reported from trials conducted in Germany and Spain with DAA coverage from national health authorities.47,48
“Real world” dataAs clinical trials demonstrated a significant reduction in waiting times to kidney transplantation with HCV viremic donors, many centers have utilized this approach in a “real world” setting.49–53 In case series reporting non-clinical trial experiences, DAAs are administered in a delayed fashion as centers must apply to patient insurance for medication coverage and DAAs are often not available until several months after transplantation. While 98% patients reported achieved SVR-12 with first-line treatment (2 required retreatment to achieve SVR), prolonged HCV viremia has led to a notable increase in complications compared to the clinical trial experience, including fibrosing cholestatic hepatitis,53 elevated rates of opportunistic infections (BK and CMV),49 as well as an increased frequency of de novo DSA.49 However, while the total number of HCV negative patients receiving a HCV viremic donor is still quite small, it is possible that additional, rare complications are yet to be discovered as well as that currently observed adverse events may not actually be that frequent in a larger cohort, highlighting the necessity of closely monitoring and reporting outcomes in this population.
ConsiderationsDetection of rare complications and clarification of the risks associated with HCV viremic donors is of concern to the transplant community. To address this, COAUTHOR (Consortium to study outcomes after transplanting HCV viremic kidneys into HCV negative recipients) was formed and represents transplants performed at the Massachusetts General Hospital, University of Pennsylvania, University of Tennessee Health Science Center, Methodist University Hospital, and Vanderbilt University Medical Center for a total of 1864 recipients of HCV viremic kidneys. In their combined cohort,54 receipt of a HCV viremic donor was not associated with an increased incidence of low level BK viremia (>1000copies/ml, HR 1.26, 95% CI 0.87–1.84) nor was timing of HCV treatment, but HCV viremic donors were associated with high viral load BK viremia (>10,000copies/ml, HR 1.69, 95% CI 1.04–2.74), highlighting the need for adequate numbers of patients to accurately quantify the risks associated with HCV viremic donors.
A look toward the futureWhile transplantation with a HCV viremic donor followed by DAA treatment is still cost effective compared to dialysis,55 the added expense of DAAs to total procedural costs can be a barrier. In studies utilizing patient insurance to cover DAA costs, prior authorization is nearly universally required and appeals are common; frequently patients covered by Medicaid experience longer times to insurance approval56 but delays are not infrequent with commercial payors as well.57 Fortunately, direct patient out of pocket costs are usually minimal. Likewise, there is an additional burden to patients in taking extra medications for the typical 12 weeks required to achieve SVR. As the hepatitis C field has moved toward shorter treatment regimens (8 weeks’ duration)58 so has HCV viremic donor transplantation and specifically there has been interest in short-course prophylaxis strategies. A four-week prophylactic course of glecaprevir/pibrentasvir has been shown to be effective in preventing HCV transmission from viremic donors in a cohort of 10 patients.59 Even shorter duration approaches have been tried. Gupta et al. have examined two dose and four dose regimens using sofosbuvir/velpatasvir; overall viral transmission was 12% and only 83% achieved SVR once treated with a full standard regimen; 33% of those HCV infected required retreatment with a second-line regimen.60 In a trial of 7 days prophylaxis with sofosbuvir/velpatasvir, transmission rates were reduced to 4% but still not zero.61 The optimal duration of prophylactic therapy to completely prevent HCV transmission remains to be determined but is likely somewhere between 7 days and 4 weeks.
ConclusionsOver the past 30 years, use of HCV-infected donors has gone from somewhat controversial to routine practice, even in HCV-uninfected recipients. This necessary response to the organ shortage has been made possible due to more effective treatments for HCV infection. As clinical practice continues to evolve, identifying the ideal duration of DAA therapy in HCV naïve recipients remains an important clinical question, as does ensuring equitable access to HCVD+ for all transplant recipients.
Conflict of interestThe authors have no relevant financial conflicts of interest to report.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.