Mitochondrial diseases are a phenotype and genotype heterogeneous group of disorders that typically have a multisystemic involvement. The m.3243A>G pathogenic variant is the most frequent mitochondrial DNA defect, and it causes several different clinical syndromes, such as mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS), and the maternally inherited diabetes and deafness (MIDD) syndromes.
Not frequently reported, renal involvement in these diseases is probably underestimated, yet it increases morbidity. It generally manifests as subnephrotic proteinuria and progressive deterioration of kidney function. Adult presentation of mitochondrial diseases is hard to recognize, especially in oligosymptomatic patients or those with exclusive kidney involvement. However, suspicion should always arise when family history, particularly on the maternal side, and multisystemic symptoms, most often of the central nervous system and skeletal muscles, are present.
In this review we discuss the clinical diagnosis and approach of patients with renal manifestations in the context of the mtDNA m.3243A>G pathogenic variant.
Las enfermedades mitocondriales son un grupo heterogéneo de trastornos fenotípicos y genotípicos que típicamente tienen una afectación multisistémica. La variante patógena m.3243A>G es el defecto del ADN mitocondrial más frecuente y causa varios síndromes clínicos diferentes, como la síndrome MELAS (mitochondrial encephalopathy, lactic acidosis and stroke-like episodes), y la síndrome MIDD (maternally inherited diabetes and deafness).
Aunque no se informa con frecuencia, la afectación renal en estas enfermedades probablemente se subestima, pero aumenta la morbilidad. Generalmente se manifiesta como proteinuria subnefrótica y deterioro progresivo de la función renal. La presentación en adultos de las enfermedades mitocondriales es difícil de reconocer, especialmente en pacientes oligosintomáticos o con afectación renal exclusiva. Sin embargo, la sospecha siempre debe surgir cuando hay antecedentes familiares, particularmente del lado materno, y síntomas multisistémicos, con mayor frecuencia del sistema nervioso central y los músculos esqueléticos.
En esta revisión discutimos el diagnóstico clínico y el abordaje de pacientes con manifestaciones renales en el contexto de la variante patogénica mtDNA m.3243A>G.
Mitochondrial diseases are a clinically and genetically heterogeneous group of disorders. The underlying dysfunction of the mitochondrial electron transport chain and oxidative phosphorylation is caused by variants of genes encoding mitochondrial proteins. Whilst each of these variants is individually rare, the estimated prevalence of all mitochondrial diseases is approximately 1:8500 (95% C.I.),1 making up quite a frequent group within rare diseases.
Kidney involvement in these diseases is uncommon but may be higher than current estimates since many cases are not properly diagnosed. Noteworthy, renal disease when present may significantly increase patient morbidity. Therefore, we reviewed clinical diagnosis and approach of patients with renal manifestations and genetic mitochondriopathies resulting from the most common pathogenic variant, the mtDNA m.3243A>G.
Mitochondrial genetics and pathophysiologyThe mitochondrial respiratory chain is an enzymatic complex composed of 70 polypeptides. Only 13 of those are encoded by mitochondrial DNA (mtDNA) genes, the remaining being encoded by nuclear DNA (nDNA).2,3 It is therefore understandable that this group of diseases can originate from defects in both mtDNA and nDNA, leading to different patterns of inheritance. Mitochondrial DNA includes 37 different genes, encoding not only respiratory chain polypeptides, but also RNA components necessary for intra-mitochondrial protein synthesis, such as transfer RNA (tRNA) and ribosomal RNA (rRNA).3
The m.3243A>G pathogenic variant in the leucine tRNA gene is the most frequent mtDNA defect.4
The characteristic heterogeneity of mitochondrial diseases is due to heteroplasmy, the co-existence of copies of mutated and healthy mtDNA in each cell. The percentage of wild-type mtDNA necessary to ensure normal functioning of the mitochondrial respiratory chain varies between individuals and even among different tissues.5 Tissues with a higher cellular turn-over, such as the hematopoietic precursors, tend to express less mutated mtDNA, due to a natural selection of cells with a higher percentage of wild-type mtDNA; conversely, post-mitotic cells, such as neurons and podocytes, are more easily damaged.6,7 The proportion of mutated versus wild-type mtDNA usually defines the clinical phenotype and its severity.6 This explains why the same pathogenic variant can cause different phenotypes within the same family.2–4,7
Clinical features of mitochondrial diseasesMitochondrial diseases can present both as syndromic or non-syndromic phenotypes. In the former situation, multi-organic involvement can be evident from the initial clinical presentation or it can develop along with the progression of the disease.8 Symptoms develop during the first weeks of life in one-third of patients, and by the age of two 80% of patients are clinically symptomatic.7 Nonetheless, some patients only become symptomatic during adulthood.6
The most frequently affected organs in mitochondrial diseases are those with high demand for oxidative energy, such as the central nervous system, typically the first involved, and skeletal muscle. However, because mitochondria are ubiquitous, many more organs and tissues may be affected by these diseases, such as the heart, lungs, endocrine organs, peripheral nerves, eyes, ears, gastrointestinal tract, bones, skin, and kidneys, the main object of this review.8
Frequent clinical manifestations include encephalopathy, myopathy, seizures, development delay, ophthalmoplegia, retinal degeneration, cardiomyopathy, sensorineural deafness, diabetes mellitus, hypoparathyroidism and liver disease.6 These symptoms can cluster in different ways, depending on the underlying mutation or disease.
Kidney involvement in mitochondrial diseasesSymptoms related to renal disease may be unreported because they are frequently subclinical or outweighed by more severe neurological symptoms. However, in a series of 42 children diagnosed with a mitochondrial disease, when all renal disorders were thoroughly analyzed, half of those patients presented with some kind of kidney involvement.9
Kidney involvement in mitochondrial diseases may be classified according to the predominance of renal disease in the syndrome (it can dominate the phenotype or it can be a non-dominant characteristic), and whether it constitutes a primary manifestation of the disease or is secondary to the involvement of other organs and tissues.8 Kidney involvement can be secondary to heart disease (due to hemodynamic and nonhemodynamic pathways occurring in cardio-renal syndrome), pancreatic disease (due to mitochondrial diabetes), or mitochondrial myopathy (secondary to rhabdomyolysis). Primary kidney disease can occur as different tubulopathies, tubulointerstitial nephritis, cystic kidney disease, nephrocalcinosis, glomerular disease (more often as focal segmental glomerulosclerosis – FSGS), or neoplasia.7,8,10
Most frequently, renal symptoms are part of a multisystemic disorder. There are, however, exceptions in which kidney dysfunction may be the only manifestation at clinical presentation, as in some mtDNA pathogenic variants and some cases of CoQ10 deficiency.7
Renal tubular disordersKidney involvement is most frequently expressed as tubular disorders. Being responsible for the reabsorption of solutes, the tubular cells play a very high energy-demanding role and are rich in mitochondria. The severity of the tubular defect may range from partial defects with an isolated wasting of electrolytes (isolated renal tubular acidosis, isolated hypomagnesemia, hypercalciuria, glycosuria, or Bartter-like phenotype) to its most severe form, a complete Fanconi syndrome.6,7
Glomerular diseasesAlthough less frequently, the glomerulus may also be involved. Podocytes are post-mitotic cells, rich in mitochondria and highly dependent on oxidative energy. Mitochondrial impairment in these cells will result in functional and structural alterations, leading to glomerular sclerotic lesions. Glomerular involvement may translate clinically by non-responsive to immunosuppression proteinuria, and progressive renal deterioration. Two identities are of particular interest when referring to glomerular disorder in mitochondrial diseases, the m.3243A>G pathogenic variant, and the CoQ10 biosynthesis defects, the latter with the particularity of responding to treatment with oral CoQ10 supplementation.6,7
The mtDNA m.3243A>G pathogenic variantThe mtDNA m.3243A>G pathogenic variant is a point mutation in the MT-TL1 gene (one of the 22 mtDNA genes), that triggers a disturbance in the translation of the mitochondrial proteins of the respiratory chain, resulting in decreased ATP synthesis.4 As mentioned above, the m.3243A>G in the leucine tRNA gene is the most frequent mtDNA defect, with an estimated prevalence of 0.06% in the general population.11 Initially described in patients with MELAS, it is known today that it can produce several other phenotypes and that the MELAS disease itself can be caused by other pathogenic variants (more than 30 have been reported).4,6 Regarding MELAS, the MT-TL1 gene is responsible for more than 80% of the cases.1
In addition to MELAS, other clinical syndromes associated with this mtDNA pathogenic variant include maternally inherited diabetes and deafness (MIDD) and chronic progressive external ophthalmoplegia (CPEO). The physiopathological reasons that lead to these different expressions of the same mutation are unknown but are probably due to the phenomenon of mtDNA heteroplasmy and mitotic segregation.12
MELAS (mitochondrial encephalopathy, lactic acidosis and stroke-like episodes)MELAS is a multi-organ disease, characterized by the cardinal triad of stroke-like episodes, seizures, and high levels of serum lactate. The age of onset for the majority of individuals (65–76%) is between 2 and 20 years.2 Some other features include myopathy, dementia (resulting from the underlying neurological dysfunction and gradual accumulation of neurological deficits caused by the stroke-like episodes), cardiomyopathy, short stature, sensorineural deafness, recurrent headaches, altered consciousness, diabetes mellitus, developmental delay and recurrent vomiting .13 Renal involvement may also be present and it will be detailed further on.
MIDD (maternally inherited diabetes and deafness)Patients with MIDD present with diabetes and progressive sensorineural deafness, as well as kidney involvement, mostly in the form of FSGS. Diabetes presentation depends on the level of insulin deficiency but, in most patients, it develops insidiously and mimics type 2 diabetes mellitus. Almost half of the affected individuals need insulin within 10 years of treatment, and metformin should be avoided, due to the risk of lactic acidosis.14 This disease should be considered as a differential diagnosis in patients with diabetes and renal disease, as its prevalence among these individuals is estimated to be 0.5–2.8%.15 Some features such as a maternal family history of diabetes and deafness, low or normal body mass index (BMI), retinal dystrophy and absence of diabetic retinopathy should raise suspicion over the possibility of MIDD.14,15
CPEO (chronic progressive external ophthalmoplegia)CPEO is characterized by symmetrical ptosis and weakness of extraocular muscles, with decreased eye motility. In contrast to MELAS syndrome, kidney involvement in CPEO patients is rarely reported, although FSGS has been described.16
Kidney involvement in the m.3243A>G pathogenic variantAlthough kidney involvement is not the most usual characteristic in patients with the mtDNA m.3243A>G variant, several of them have been diagnosed with renal disease. In an analysis of 117 individuals with mitochondrial disorders, 75 patients (64%) had the m.3243A>G variant, and around one-third of these presented with kidney disease. Given a similar prevalence of kidney involvement in the other less frequent mitochondrial genetic abnormalities, this is likely the most common cause of mitochondrial disorders with kidney involvement.17 Patients are usually diagnosed during the second or third decade of life with proteinuria below the nephrotic range, and progressive deterioration of renal function. Kidney involvement seems to be more frequent in female patients with the m3243A>G variant.7
From the histologic point of view, the most common alteration is FSGS (present in 78% of cases with MELAS)12; electronic microscopy often shows abnormal mitochondria with a decreased number of ridges in severely effaced pedicels, but also in the tubular cells; cells may show depletion of mitochondria or a compensatory proliferation of these organelles.6,18 Some studies report peculiar arterial and arteriolar lesions (hyaline cytoplasmic condensation in smooth muscle), particularly of the afferent arterioles.5,10,19 They consist of smooth muscle cells crammed with enlarged and abnormal mitochondria, alternating with smooth muscle cells showing pyknosis and shrinkage. A few cases of tubulointerstitial nephritis have also been reported.7
DiagnosisThe diagnosis of mitochondrial disease in a patient who presents with kidney disease can be a real challenge, due to the rarity of the etiology and the diversity of phenotypic presentation. Nevertheless, this diagnosis is a fundamental step in the management of the patient, as it concerns treatment (such as preventing the exposure of these patients to immunosuppressive therapy, as in idiopathic FGSG), family screening, and genetic counseling.
Mitochondrial disease should therefore be suspected whenever there is a family history of similar clinical presentation, a task that is naturally hampered by phenotypic variability, and by the different patterns of inheritance of mitochondrial diseases – if the pathogenic variant is located in mtDNA the family history will be present on the maternal side of the family, whereas if the mutation is located in the nDNA it can follow an autosomal dominant, autosomal recessive or X-linked pattern of inheritance.20 Suspicion should also arise if there is systemic involvement in addition to kidney disease, especially if it affects the central nervous system and skeletal muscle. These are indeed the most commonly affected systems, although this may not be immediately present with the kidney disease, so careful monitoring to multi-organic involvement is essential.3
Metabolic acidosis is also frequently found in most of these individuals. However, in those patients with predominantly renal disease, serum lactates are generally normal, whereas urine lactate may be increased in those with a tubular disease, but rarely in glomerulopathies.6
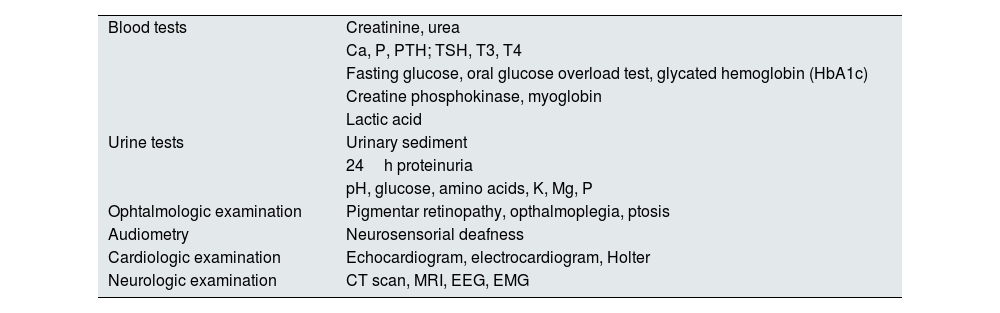
Table 1 summarizes the complementary tests that should be performed not only when suspecting a mitochondrial disease, but also during the follow-up of a patient with a confirmed diagnosis since some features can only become apparent with time.
Complementary exams for patients with suspected or confirmed mitochondrial disease.
| Blood tests | Creatinine, urea |
| Ca, P, PTH; TSH, T3, T4 | |
| Fasting glucose, oral glucose overload test, glycated hemoglobin (HbA1c) | |
| Creatine phosphokinase, myoglobin | |
| Lactic acid | |
| Urine tests | Urinary sediment |
| 24h proteinuria | |
| pH, glucose, amino acids, K, Mg, P | |
| Ophtalmologic examination | Pigmentar retinopathy, opthalmoplegia, ptosis |
| Audiometry | Neurosensorial deafness |
| Cardiologic examination | Echocardiogram, electrocardiogram, Holter |
| Neurologic examination | CT scan, MRI, EEG, EMG |
Ca: calcium; P: phosphorus; PTH: parathormone; K: potassium; Mg: magnesium; CT scan: computed tomography; MRI: magnetic resonance imaging; EEG: electroencephalography; EMG: electromyography.
The detection of genetic alterations is nowadays a fast and easily accessible procedure with next-generation sequencing techniques that include panels of genes aimed at detecting mitochondrial diseases, but that also allow the sequencing of the whole mitochondrial genome and analysis of heteroplasmy.21 Target testing of specific mtDNA pathogenic variants can also be performed by Sanger sequencing. The study sample should preferably be obtained from the affected organs and not from peripheral blood, since the levels of mutated mtDNA are lower in the blood sample, because of the high mitotic index of the hematic cells. Regarding kidney disease, DNA samples should be obtained from the urinary sediment, which has a higher concentration of mtDNA from the tubular epithelial cells.6,22 It should be noted, however, that the ratio between mutated and normal mtDNA does not necessarily correlate with the severity of kidney disease.22
Kidney biopsy, although not specific, usually reveals some characteristic alterations of mitochondrial diseases. Moreover, the biopsy is also important for the differential diagnosis with other diseases, such as Alport syndrome. Since a high percentage of patients with the mtDNA m.3243A>G pathogenic variant with renal involvement also suffer from sensorineural hearing loss, these patients may be confounded with Alport patients. However, in these cases, hematuria is more frequent, electron microscopy will present different characteristics and genetics will provide definitive diagnosis.7
Treatment of CKD patients with mitochondriopathiesGeneral measuresThere is not nowadays any evidence-based treatment for mitochondrial disease, partly owing to the difficulty in conducting large-scale trials in such a heterogeneous condition. Current treatment options for MELAS and other mitochondrial cytopathies are largely supportive, including symptomatic management and supplementation with antioxidants and cofactors, such as l-arginine that should be used for treatment and prevention of stroke-like episodes.13
Approaches used, even though with limited success, include reactive oxygen species scavengers (e.g. vitamin E, glutathione), cofactors to enhance metabolism and ATP synthesis (e.g. B complex and C vitamins, l-carnitine, folic acid), and electron acceptors (e.g. C and E vitamins, succinate).23 CoQ10, riboflavin, and α-lipoic acid should be offered to mitochondrial disease patients. Folinic acid should be considered if patients develop central nervous system manifestations and l-carnitine when there is a documented deficiency.21
Regular exercise has been advocated, with benefits from both endurance and resistance training in exercise-induced mitochondrial biogenesis.21,22 Avoidance of drugs potentially toxic to mitochondria (e.g., metformin, valproic acid, aminoglycosides, linezolid, and alcohol) is important. Smoking should also be evicted, as it can aggravate mitochondrial dysfunction.13
Gene therapy to introduce wild-type tRNA into an affected cell population may be possible in the future.18
Dialysis and mitochondriopathiesWhen CKD progresses to end-stage renal disease (ESRD), renal replacement techniques (RRT) may be considered, after a careful evaluation of the patient's prognosis. Notwithstanding a paucity of data about the role of RRT, several cases of mitochondriopathy patients with CKD starting hemodialysis have been reported.5,24–27 One of these reports describes a childhood mitochondriopathy successfully managed with peritoneal dialysis (PD), highlighting the role of dialysate containing both bicarbonate and lactate versus one containing exclusively lactate in managing patient's symptoms.28 This report suggests that PD solution buffered with less lactate (preferably with a bicarbonate only solution) is a preferential choice in PD patients with mitochondrial cytopathies.
Kidney transplantation and mitochondriopathiesDespite the shortened life expectancy, successful kidney transplantation allows for an improvement in the quality and life expectancy of these patients. At least a total of 29 transplant patients with the m.3243A>G pathogenic variant were described in the literature.25,27,29 In an analysis of 35 post-solid organ transplant patients with mitochondriopathies, 12 patients received a kidney transplant, 7 of which had the m.3243A>G variant (58%). Survival was 100% for kidney transplant patients, with a range of follow-up between 1 and 12 years (mean and median 5 years).29
Due to immunosuppression, particularly corticotherapy, progression of, or new-onset diabetes is a frequent complication in transplant patients with mitochondriopathies.27
Conversely, a clinical trial published in 2018 studied the advantages of performing immunosuppression with the mechanistic target of rapamycin (mTOR) inhibitors, after documenting an overactivation of this pathway in fibroblasts from 4 patients affected with mitochondrial disease.25 The patients included in the study had undergone kidney transplantation, with no complications related to immunosuppression (with calcineurin inhibitors), but with worsening general condition or cachexia, secondary to the mitochondriopathy. In these patients, a therapeutic switch was performed to rapamycin. The study focuses both on the cellular effects of this drug – with attenuation of the premature senescence of fibroblasts and recovery of the morphology and membrane potential of the mitochondria – and on the general condition of the patients. After a few weeks, they had a marked improvement in their general condition (not related to renal function improvement, since the glomerular filtration rate remained stable during the switch), as well as in their metabolic profile. So, despite the main aim of the study being related to post-transplant patients in need of immunosuppression, the possibility of using the inhibition of the mTOR pathway in mitochondrial patients with documented mTOR hyperactivation was raised.25
Genetic counseling and pregnancy in CKD patients with mitochondriopathiesGenetic counseling is an important component of patient management, as it provides individuals and their families with information regarding the prognosis, the recurrence risk for family members, as well as the existing strategies for the prevention of maternal transmission of mtDNA pathogenic variants. However, in the case of mitochondriopathies, this poses as particularly challenging. This is so, not only because of the possibility of different patterns of inheritance according to the genome affected (nuclear or mitochondrial), but also due to heteroplasmy and the bottleneck effect. The heteroplasmic state implies that there are both mutated and wild-type mtDNA copies in a cell and the penetrance (threshold effect) and disease severity depend on the proportion of mutated versus wild-type genome present. Additionally, the bottleneck effect, by which there is a marked reduction in mtDNA copy number during early oogenesis, can likewise cause a significant shift in the percentage of mutated mtDNA transmitted to the offspring. Baring in mind this characteristics, one can understand that it is very difficult to predict the phenotypic consequences for the offspring of a woman carrying a mtDNA mutation.20
Another important question to address with women with mitochondriopathies and kidney disease that plan to get pregnant are the risks to the health of both mother and fetus. Pregnancy is associated with a progression of kidney disease and an unpredictable neurological outcome for the mother. In fact, the gestational period is often complicated in patients with MELAS, namely with the development of hypertensive diseases, such as pre-eclampsia, or gestational diabetes. Also, medical and obstetric complications such as postpartum hemorrhage, pulmonary edema, new onset of neurological symptoms or worsening of the central nervous system function, hyperkalemia, and metabolic acidosis are common.30,31
Moreover, the health of the fetus is also called into question, firstly by the increased risk of preterm birth – associated with a reduced number of nephrons at birth and, therefore, with a greater risk of developing hypertension and CKD, besides a higher risk of intellectual deficits. Additionally, the child will inherit the mother's mitochondrial disease, with the phenotypic expression and severity not possibly to be determined. The combination of mitochondrial disease with the high risk of preterm birth can result in a poor outcome for the child's health.31
Counseling a pregnant woman in these conditions proves to be a difficult task, with the need to disclose the involved risks, both for patient health and for the fetus, as well as the short- and long-term implications that may arise if it is decided to let the pregnancy follow its course.31
OutcomesPatients carrying the m.3243A>G pathogenic variant, namely MELAS patients, have a shortened life expectancy, with progressive neurological deterioration, and an estimated overall median survival of 16.9 years from the onset of neurological symptoms.32 As far as we are aware, there are no systematic studies concerning the impact of renal involvement in the outcome of these mitochondriopathies. Regarding the severity of kidney disease, it is theorized that it worsens as the heteroplasmy rate in the kidneys increases.
New perspectivesThe extent to which mitochondrial damage contributes to kidney disease is uncertain, and a cause–effect relationship is yet to be established. However, the contribution of mitochondria dysfunction in the pathogenesis of multiple types of kidney disease is well recognized. This includes not only kidney disease secondary to mitochondrial genetic defects, but also acute kidney disease, CKD, renal tumors, aging, and transplant nephropathy.18,33 For this reason, treatments that specifically target mitochondria are being tested. They seem to alleviate renal injury in experimental animal models, and so represent a promising therapeutic option for patients with kidney disease.18
ConclusionsMitochondrial diseases are a heterogeneous group, which usually become symptomatic within the first years of life. The m.3243A>G pathogenic variant in the leucine tRNA gene is the most frequent mtDNA defect, and it causes several clinical syndromes with different phenotypes, such as MELAS and MIDD syndromes. Renal involvement in these diseases is not very common, but it can represent an important aggravating factor in the morbidity of these patients. It typically manifests as FSGS lesions, with subnephrotic range proteinuria and progressive deterioration of kidney function. Renal replacement techniques may be necessary. There are some important considerations to be aware of in renal transplant patients, namely the adverse effects of concomitant therapy. Concerning immunosuppression, mTOR inhibitors may theoretically provide a potential benefit. Current treatment options for mitochondrial cytopathies are largely supportive, including symptomatic management and supplementation with antioxidants and cofactors.
Given the high phenotypic variability of these diseases, the diagnosis requires a high level of suspicion, especially if only renal symptoms are present. Suspicion should arise if there is a family history of a similar clinical presentation, particularly on the maternal side, and if there is a multisystemic involvement, most often of the central nervous system and skeletal muscles. Genetic counseling is an important component of patient management.
Authors’ contributionsConception and design was performed by Clara Bacelar, Filipa Ferreira and Ana Teresa Nunes. The first draft of this paper was written by Clara Bacelar and Filipa Ferreira, and all authors commented on subsequent versions. All authors performed a critical revision of the article and approved the final text.
FundingNone declared.
Conflicts of interestThe authors have no conflicts of interest to declare.