Dear Editor,
Amyloidoses are a group of life-threatening diseases characterized by deposition of fibrillar misfolded proteins with an antiparallel beta-sheet structure.1 Renal Amyloidosis is determined by extracellular deposition of amyloid fibrils within kidney compartments. Clinically evident renal involvement is presented as proteinuria or nephrotic syndrome.2 Systemic reactive (AA) amyloidosis, leading to renal failure, is a severe complication of most hereditary periodic fever syndromes. We report the occurrence of renal AA amyloidosis causing severe nephrotic syndrome in a young Italian man affected with Hyper-IgD Syndrome (HIDS).
CASE REPORT
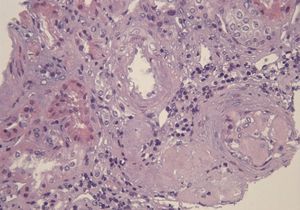
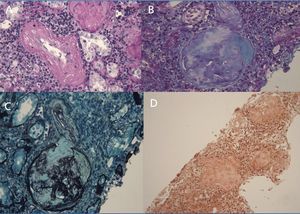
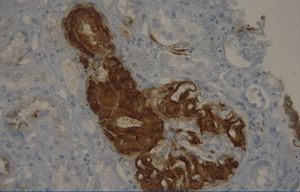
A 29-year-old man of southern Italian ancestry, was admitted to the hospital. Since the first months of life he had experienced characteristic febrile attacks associated with sore throat, myalgias, vomiting and diarrhoea. Later the attacks of fever reduced their frequency but in the last year there was an increased frequency of febrile episodes with proteinuria in nephrotic range. On admission his blood pressure was 150/80mmHg and temperature 36.5°C. Heart sounds were clear with regular sinus rhythm and pulse rate was 88 beats/min. Physical examination showed normal findings and no symptoms suggestive of respiratory, abdominal or urinary infection were apparent. Chest radiography and abdominal ultrasonography scan revealed no abnormalities. Laboratory investigations showed proteinuria 9.17g/day, a raised erythrocyte sedimentation rate, a normal C-reactive protein, a total leukocyte count of 12.500/mm3, serum amyloid 3.67mg/L, serum IgD 233UI/ml (normal range 0-100), creatinine 1.09mg/dl. Immunological tests and other laboratory parameters resulted negative or within normal limits. Kidney biopsy: among 28 glomeruli, 18 showed a weakly eosinophilic amorphous material infiltrating the mesangium (Figure 1 and Figure 2). After positive Congo red staining, the deposits revealed apple-green birefringence under polarized light, consistent with the presence of amyloid. On immunochemistry, the amyloid deposits were negative for antibodies against kappa and lambda chains (Figure 3). Clinical and laboratory findings suggested diagnosis of systemic reactive amyloidosis. The research for MEFV, for Familial Mediterranean Fever (FMF) and TNFRSF1A, for Tumor Necrosis Factor Receptor-Associated Periodic Syndrome (TRAPS) mutations was negative; the research for 2 mevalonate kinase (MVK) mutations was positive in heterozygosis, diagnosing the HIDS. The patient started therapy with Anakinra, Interleukin -1 antagonist.
DISCUSSION
Renal amyloidosis comprise a spectrum of vascular, glomerular, and tubulointerstitial deposition. The reason for the preferential localization to one or the other compartment is not well established. It seems very likely that the varying chemicophysical properties of the amyloid fibrils determine the tropism. Currently, the distribution patterns do not aid in the management of the patients.3 At least 25 different precursor proteins are known and are associated with a variety of inflammatory, immune, infectious, and hereditary conditions. Most renal amyloidosis is either the result of primary fibrillar deposits of immunoglobulin light chains known as amyloid L (AL) or secondary to fibrillar deposits of serum amyloid A (AA) protein fragments. Renal involvement can be found in some monogenic diseases, the hereditary periodic fever syndromes, which present with recurrent inflammation and unexplained fevers as part of their phenotype. Familial Mediterranean Fever (FMF) is the most widely known and the most prevalent of these inherited disorders.4 The most dreaded complication of untreated FMF is amyloidosis, which eventuates in renal failure in as many as 20% of patients in some populations. HIDS, which mimics FMF, is a much rarer disease; it has been reported mainly in families of European ancestry, most of whom are clustered in The Netherlands. It is inherited in autosomal recessive manner. Mutations in the gene encoding MVK constitute the molecular defect in HIDS. The defective gene resides on chromosome 12q. MVK is a key enzyme in the biosynthesis of cholesterol and isoprenoid. HIDS is caused by a defect in the isoprenoid pathway; presumably, intermediary metabolites of the isoprenoid pathway (or a shortage of certain metabolites) influence the immune system in such a way that high levels of IgD are produced.5 HIDS is characterized by recurrent, self-limiting attacks of fever occurring since early childhood. Febrile episodes usually last 3–7 days and are variably associated with headache, arthralgias, lymphadenopathy, abdominal pain, diarrhoea, vomiting, and skin lesions. The diagnosis of HIDS is based on clinical criteria and elevated serum immunoglobulin D (IgD) levels (100IU/ml). Amyloidosis has been reported only rarely in HIDS. In 2006 Obici et al. did the first report to describe the occurrence of renal AA amyloidosis causing severe nephrotic syndrome in a young Italian man affected with HIDS.6
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Figure 1. Kidney biopsy
Figure 2. Kidney biopsy
Figure 3. Kidney biopsy