We estimated the relationship between routine biochemical laboratory parameters with static bone histomorphometric parameters and their high and low bone turnover capacity predictability in hemodialysis patients.
MethodIt was a single-center cross-sectional study, included 28 hemodialysis patients. The routine biochemical parameters measured including calcium, phosphorous, alkaline phosphatase, intact PTH, and 25-hydroxycholecalciferol. The histomorphometric parameters assessed were osteoblasts perimeter, osteoclast perimeter, eroded perimeter, osteoid perimeter, bone fibrosis and bone volume.
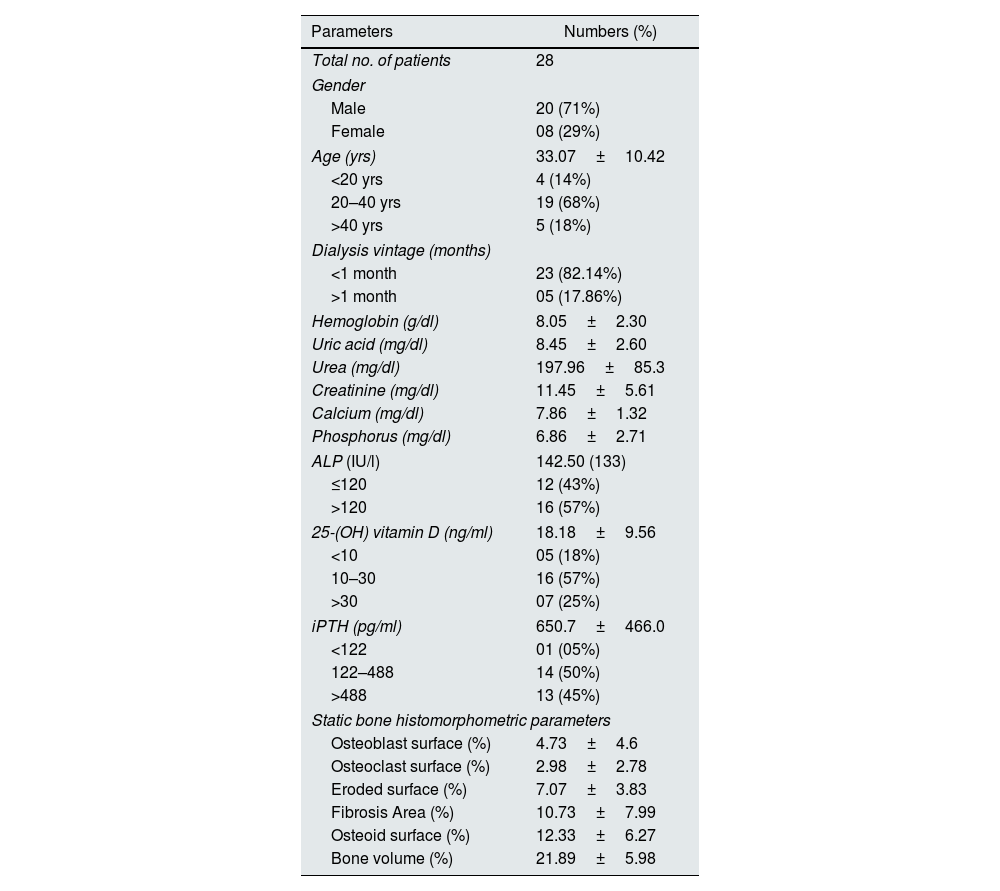
ResultTotal 28 hemodialysis patients underwent bone biopsy. Seventy percent were male, with a mean age was 33.07±10.42 yrs; serum alkaline phosphatase was 219.10±311.3IU/ml; vitamin D was 18.18±9.56ng/ml, and intact PTH was 650.7±466.0pg/ml. Intact PTH had a significant positive association with osteoblast, osteoclast, eroded surface, and osteoid perimeter. Serum alkaline phosphatase had a significant relationship with bone fibrosis (r=0.525, p-value=0.004). Intact PTH was significantly higher in females than males (1078.75±533.04 vs. 479.6±309.83; p-value=0.004). The osteoid surface was significantly high in females compared to males (p=0.038). Age had a significant impact on osteoblast and eroded surface (p=0.008 and p=0.031, respectively). Intact PTH is a reliable biomarkers for bone turnover compare to ALP (p<0.001 and p=0.554, respectively).
ConclusionIntact PTH strongly associated with bone formation, bone resorption parameters. Gender and age had significant impact on static histomorphometric parameters in our study.
Chronic kidney disease (CKD) is an increased risk of developing disturbances of bone and mineral metabolism.1 The KDIGO workgroup recommended a broader term, CKD-mineral and bone disorder (CKD-MBD) for the systemic disorder of mineral and bone metabolism due to CKD.2 The key parameter for evaluating bone turnover is the bone formation rate (BFR) and a full histomorphometric analysis of a bone biopsy with prior tetracycline labeling remains the gold standard for evaluating renal osteodystrophy that assesses bone turnover, mineralization and volume. However, bone biopsy is less frequently utilized in clinical settings. Novel imaging techniques are promising in terms of their ability to non-invasively quantify components of ROD – like dual-energy X-ray absorptiometry (DEXA) for combined bone volume and mineralization and quantitative computerized tomography (CT) for bone volume and architecture; however, they are usually not capable of measuring bone turnover.3
Bone turnover is a dynamic biological process that indicates activity of bone cells contrasts with mineralization which is a more passive physicochemical process. Although bone histomorphometry is considered the gold standard, circulating markers that can be assessed in the blood compartment are attractive alternatives for bone turnover. Direct regulator of bone formation like PTH and sclerostin, others like bone-specific alkaline phosphatase (BSAP), tartrate-resistant acid phosphatase 5b (TRAP5b), or by-products of either production or cleavage of bone collagen, like the N-terminal domain of the propeptide of procollagen 1 (P1NP) and C-terminal crosslaps (CTX).3 In routine clinical practice intact PTH and alkaline phosphatase are two biomarker frequently used. However, association of bone-specific ALP and intact PTH are different with races and even variation of PTH level with bone turnover especially in African-American has been reported.4,5 In our study we estimated the relationship between routine biochemical laboratory parameters with static bone histomorphometric parameters estimated using semi-quantitative method in hemodialysis patients in Indian subset.
MethodsIt was a single-center cross-sectional study that included CKD patients between 18 and 60 yrs and on dialysis. All bone biopsies were performed at our center between February 2020 and October 2021. A total of 28 biopsies were done through the iliac crest 2cm posterior and inferior to anterior superior iliac spine using an 8G trephine with an external/internal diameter of 4.50/3.55mm. Bone histomorphometric analyses were performed. The following were the exclusion criteria:
- •
Patients on medications that affect bone mineral metabolism; HRT (hormone replacement therapy), corticosteroid, cinacalcet, warfarin, phenytoin, and bisphosphonate within one year before enrolment.
- •
CKD patients on calcium supplements, calcium-containing phosphate binders, vitamin D or its active metabolites, and calcimimetics.
- •
Patient having bone disease/history of fractures in preceding six months.
- •
The patient has a skin infection at the biopsy site, deranged bleeding, and coagulation profile.
- •
Patients not given consent.
The biochemical profile included hemoglobin, serum urea, creatinine, uric acid, calcium, phosphorous, alkaline phosphatase (ALP), intact PTH, and 25-hydroxycholecalciferol. The LIAISON®N-TACT®PTH Gen II was used as an in vitro chemiluminescent immunoassay (CLIA) for the quantitative determination of intact human parathyroid hormone (iPTH) in serum. The test was performed on the LIAISON® Analyzer. The 25-hydroxycholecalciferol was measured by ADVIA Centaur Vitamin D immunoassay method (CLIA) and serum alkaline phosphatase by photometry.
Bone histomorphometryUn-decalcified 5-μm thick sections were stained by the Goldner trichrome method to determine static bone parameters. Histomorphometric analyses are based on the identification of cells and extracellular matrix by chemical staining within a region of interest and defined by primary parameters of areas they occupy, their boundary perimeters, or their distances from other points of reference. Image J was used which is available as a no-cost download from NIH website and is frequently updated and validated. All measurements in Image J are made on archived images and so determination of histomorphometric parameters are semi-automated rather than live (i.e., in real time from tissue sections).6
All bone histomorphometric parameters are reported in two dimensions, using standardized nomenclature. Parameters assessed included active osteoblasts per bone and osteoid perimeters or surface (ObPm/BPm, ObPm/OPm, %), active osteoclasts per bone and eroded perimeters or surface (OcPm/BPm, OcPm/EPm, %), eroded per bone perimeter or surface (EPm/BPm), osteoid per bone area (OAr/BAr) and the presence or absence of fibrosis. Patients were diagnosed as having low, normal, or high bone turnover in a semi-quantitative assessment by an experienced bone pathologist. Patients were categorized as high turnover using signs of excessive bone resorption (EPm/BPm above normal range of 0.5–3.4%), or evidence of disordered bone formation (marrow fibrosis>5%). Patients were categorized as low turnover if limited amounts of osteoid (OAr/BAr, normal range 0.23–5.83%) and without the presence of fibrosis (detailed in Supplementary File).6 Tetracycline labeling (dynamic study) was not performed in our study. The consent was taken from all included patients (Declaration of Helsinki), and the study was approved by the institute ethical committee (AIIMS/IEC/2019-20/1037).
Statistical analysis was carried out using the software program SPSS version 28.0. (SPSS Inc., Chicago, USA). Data were presented as mean±standard deviation, number (%), or median with IQR. Spearman's correlation coefficient evaluated correlations between biochemical parameters and bone histomorphometric measure. Multivariate linear regression analysis was used to determine the association between various factors. Mann–Whitney U or Kruskal–Wallis test were used to compare two or more than two groups respectively. Statistical significance was set at a p-value <0.05.
ResultsA total of 28 patients with CKD underwent bone biopsy. Basic laboratory parameters are highlighted in Table 1. The vitamin D levels were 18.18±9.56ng/ml, and around three-fourths of patients were deficient in vitamin D. Most patients had high PTH levels with a mean level of 650.7±466.0pg/ml, and around forty-five percent have more than 488pg/ml. In our study, females had significantly more PTH levels than males (1078.75±533.04 vs. 479.6±309.83; p-value=0.004). The rest of the laboratory parameters were not different between the two. There was no difference in laboratory parameters with dialysis vintage.
Baseline characteristic of the study population.
| Parameters | Numbers (%) |
|---|---|
| Total no. of patients | 28 |
| Gender | |
| Male | 20 (71%) |
| Female | 08 (29%) |
| Age (yrs) | 33.07±10.42 |
| <20 yrs | 4 (14%) |
| 20–40 yrs | 19 (68%) |
| >40 yrs | 5 (18%) |
| Dialysis vintage (months) | |
| <1 month | 23 (82.14%) |
| >1 month | 05 (17.86%) |
| Hemoglobin (g/dl) | 8.05±2.30 |
| Uric acid (mg/dl) | 8.45±2.60 |
| Urea (mg/dl) | 197.96±85.3 |
| Creatinine (mg/dl) | 11.45±5.61 |
| Calcium (mg/dl) | 7.86±1.32 |
| Phosphorus (mg/dl) | 6.86±2.71 |
| ALP (IU/l) | 142.50 (133) |
| ≤120 | 12 (43%) |
| >120 | 16 (57%) |
| 25-(OH) vitamin D (ng/ml) | 18.18±9.56 |
| <10 | 05 (18%) |
| 10–30 | 16 (57%) |
| >30 | 07 (25%) |
| iPTH (pg/ml) | 650.7±466.0 |
| <122 | 01 (05%) |
| 122–488 | 14 (50%) |
| >488 | 13 (45%) |
| Static bone histomorphometric parameters | |
| Osteoblast surface (%) | 4.73±4.6 |
| Osteoclast surface (%) | 2.98±2.78 |
| Eroded surface (%) | 7.07±3.83 |
| Fibrosis Area (%) | 10.73±7.99 |
| Osteoid surface (%) | 12.33±6.27 |
| Bone volume (%) | 21.89±5.98 |
iPTH: intact parathyroid hormone; ObPm: osteoblast perimeter; OcS: osteoclast perimeter; ES: eroded perimeter; OS: osteoid perimeter; BV: bone volume; BS: bone surface; TV: total volume; S. ALP: serum alkaline phosphatase.
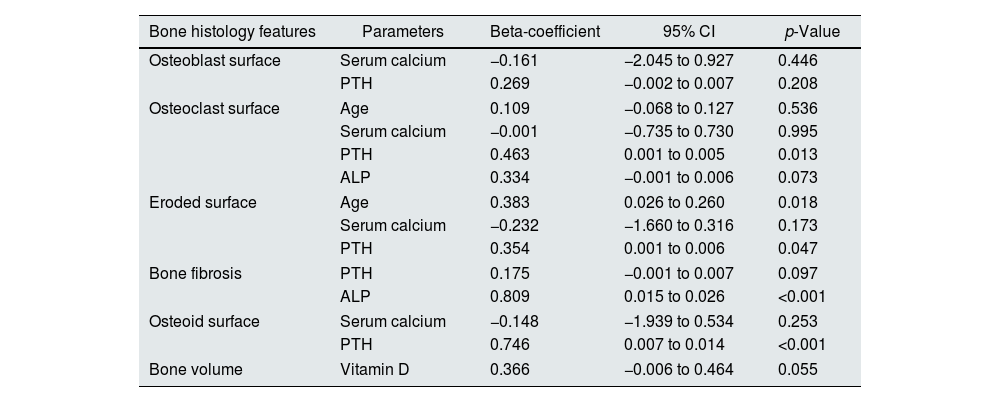
With osteoblastic activity, only PTH had a statistically significant positive relationship (r=0.466, p-value=0.013) and serum calcium had a statistically significant negative relationship (r=−0.40, p-value=0.035) shown in Table S1. With osteoclastic activity, age, PTH, and serum alkaline phosphatase had a statistically significant positive relationship (r=0.434, p-value=0.021; r=0.650, p-value<0.001; and r=0.421; p-value=0.026, respectively) and serum calcium had a statistically significant negative relationship (r=−0.387, p-value=0.042) (Table S2). In regression analysis, only intact PTH was independently associated with osteoclastic activity (beta-coefficient=0.463; p-value=0.013, Table 2).
Regression analysis of factors affecting static bone histomorphometric features.
| Bone histology features | Parameters | Beta-coefficient | 95% CI | p-Value |
|---|---|---|---|---|
| Osteoblast surface | Serum calcium | −0.161 | −2.045 to 0.927 | 0.446 |
| PTH | 0.269 | −0.002 to 0.007 | 0.208 | |
| Osteoclast surface | Age | 0.109 | −0.068 to 0.127 | 0.536 |
| Serum calcium | −0.001 | −0.735 to 0.730 | 0.995 | |
| PTH | 0.463 | 0.001 to 0.005 | 0.013 | |
| ALP | 0.334 | −0.001 to 0.006 | 0.073 | |
| Eroded surface | Age | 0.383 | 0.026 to 0.260 | 0.018 |
| Serum calcium | −0.232 | −1.660 to 0.316 | 0.173 | |
| PTH | 0.354 | 0.001 to 0.006 | 0.047 | |
| Bone fibrosis | PTH | 0.175 | −0.001 to 0.007 | 0.097 |
| ALP | 0.809 | 0.015 to 0.026 | <0.001 | |
| Osteoid surface | Serum calcium | −0.148 | −1.939 to 0.534 | 0.253 |
| PTH | 0.746 | 0.007 to 0.014 | <0.001 | |
| Bone volume | Vitamin D | 0.366 | −0.006 to 0.464 | 0.055 |
PTH: parathyroid hormone; ALP: serum alkaline phosphatase; CI: confidence interval; p-value <0.05.
With eroded surface, age and PTH have a statistically significant positive relationship (r=0.418, p-value=0.027 and r=0.628, p-value<0.001, respectively) and serum calcium had a statistically significant negative relationship (r=−0.380, p-value=0.046) (Table S3). Age and intact PTH were independently associated with the eroded bone surface (beta-coefficient=0.383, p-value=0.018 and beta-coefficient=0.354, p-value=0.047, respectively, Table 2). With osteoid surface, intact PTH had a statistically significant positive relationship (r=0.761, p-value<0.001) and serum calcium had a statistically significant negative relationship (r=−0.459, p-value=0.014) (Table S4). Only PTH was independently associated with osteoid surface area (beta-coefficient=0.746, p-value=<0.001, Table 2).
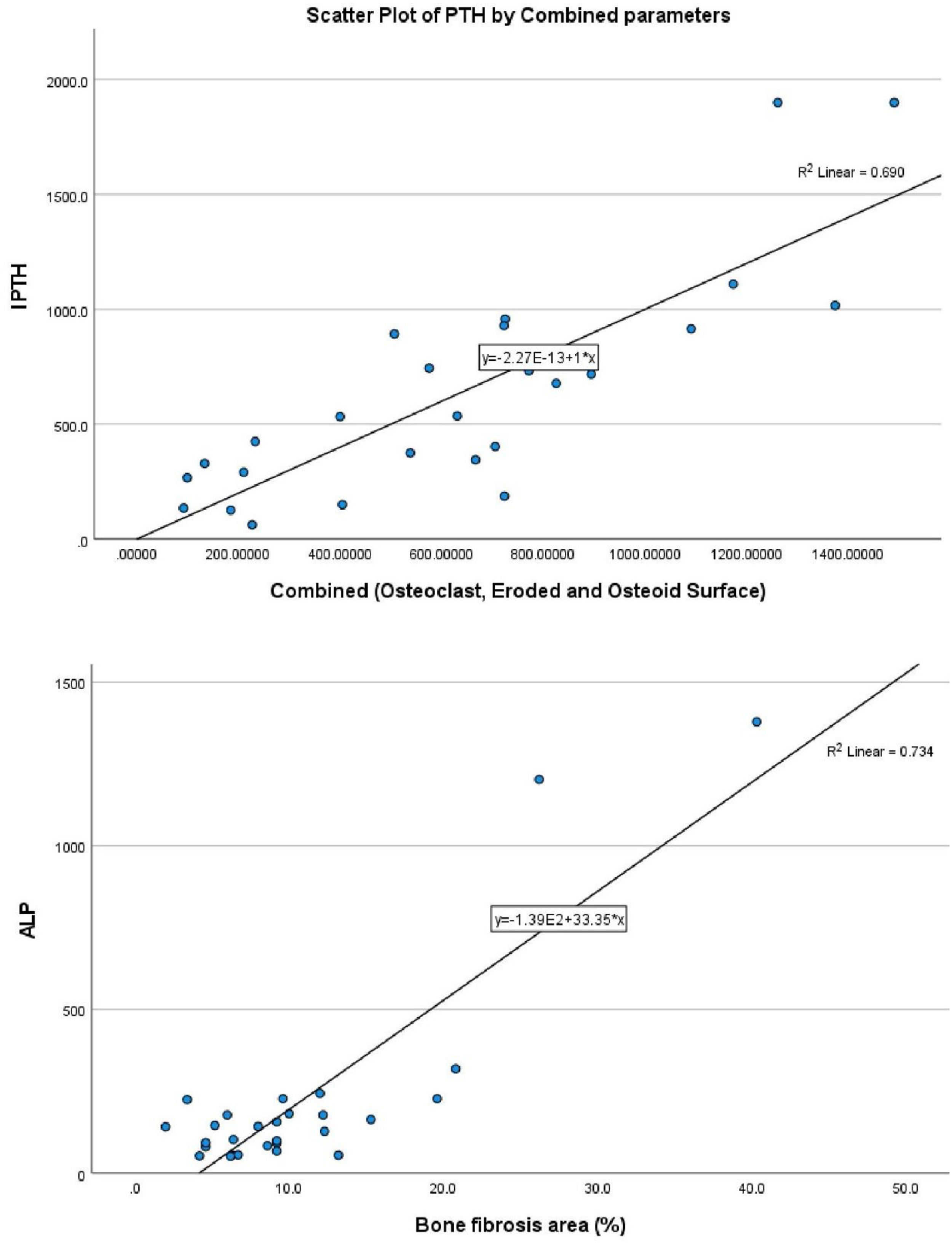
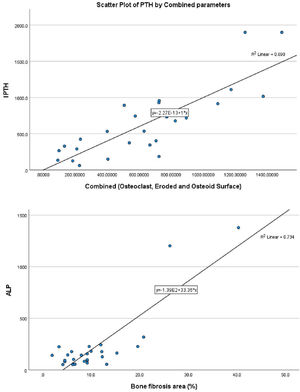
With bone fibrosis, PTH and serum alkaline phosphatase has a statistically significant positive relationship (r=0.583, p-value=0.001 and r=0.525, p-value=0.004, respectively, Table S5). Only ALP was independently associated with bone fibrosis (beta-coefficient=0.809, p-value=<0.001, Table 2 and Fig. 1B). With bone volume, vitamin D has a statistically significant positive relationship (r=0.385, p-value<0.043) (Tables S6 and 2). However, vitamin D was not independently related to bone volume (beta-coefficient=0.366, p-value=0.055).
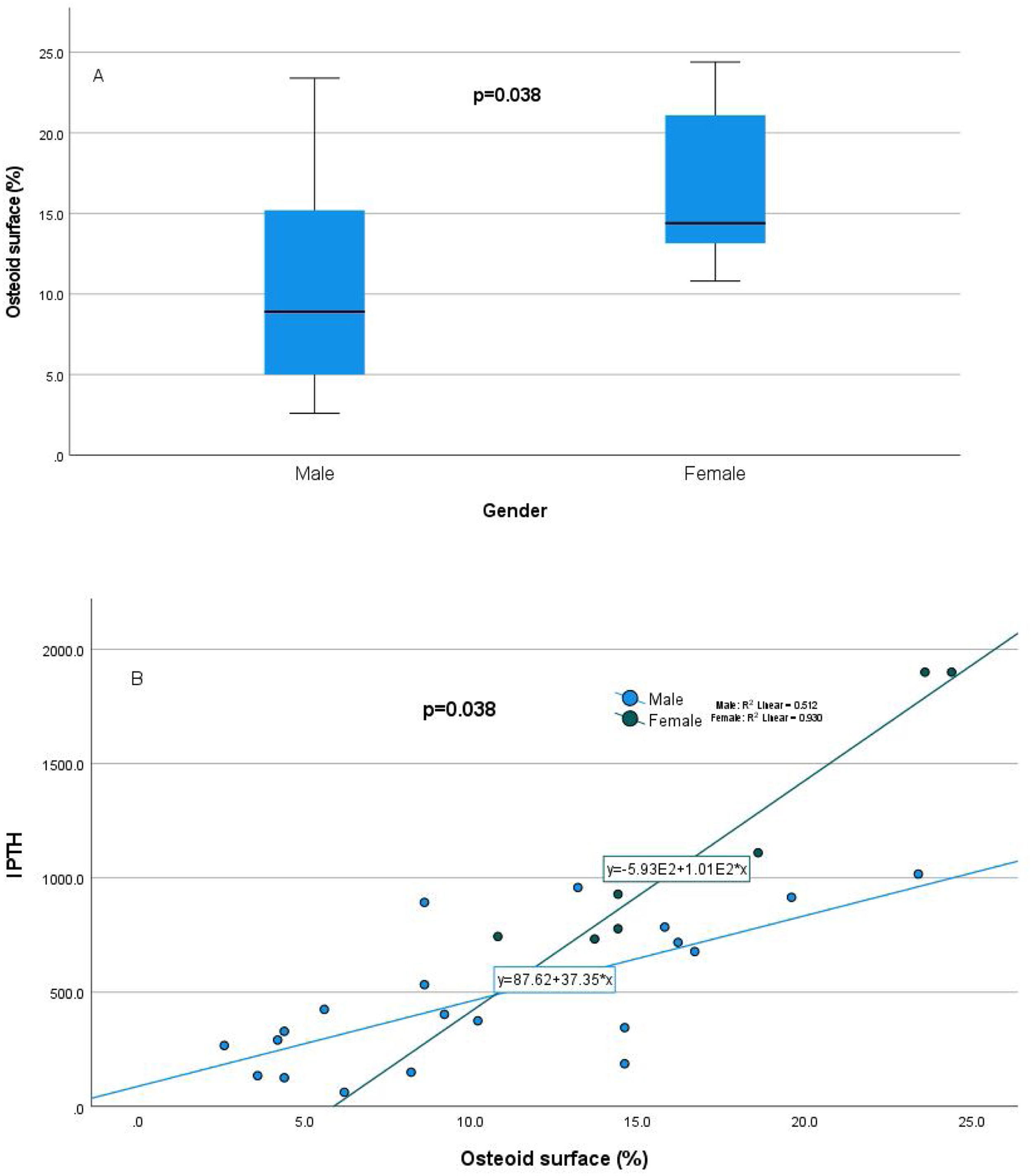
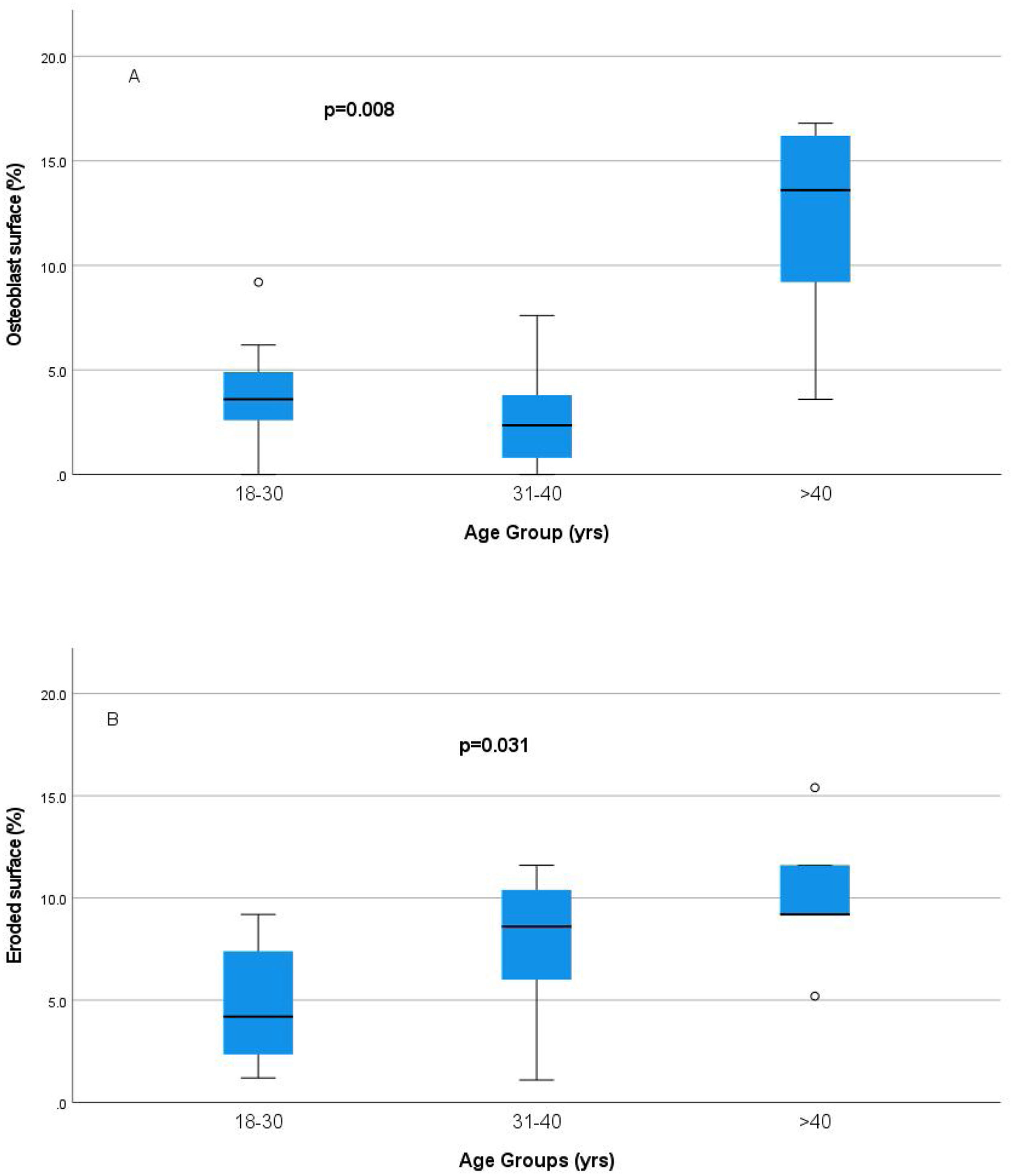
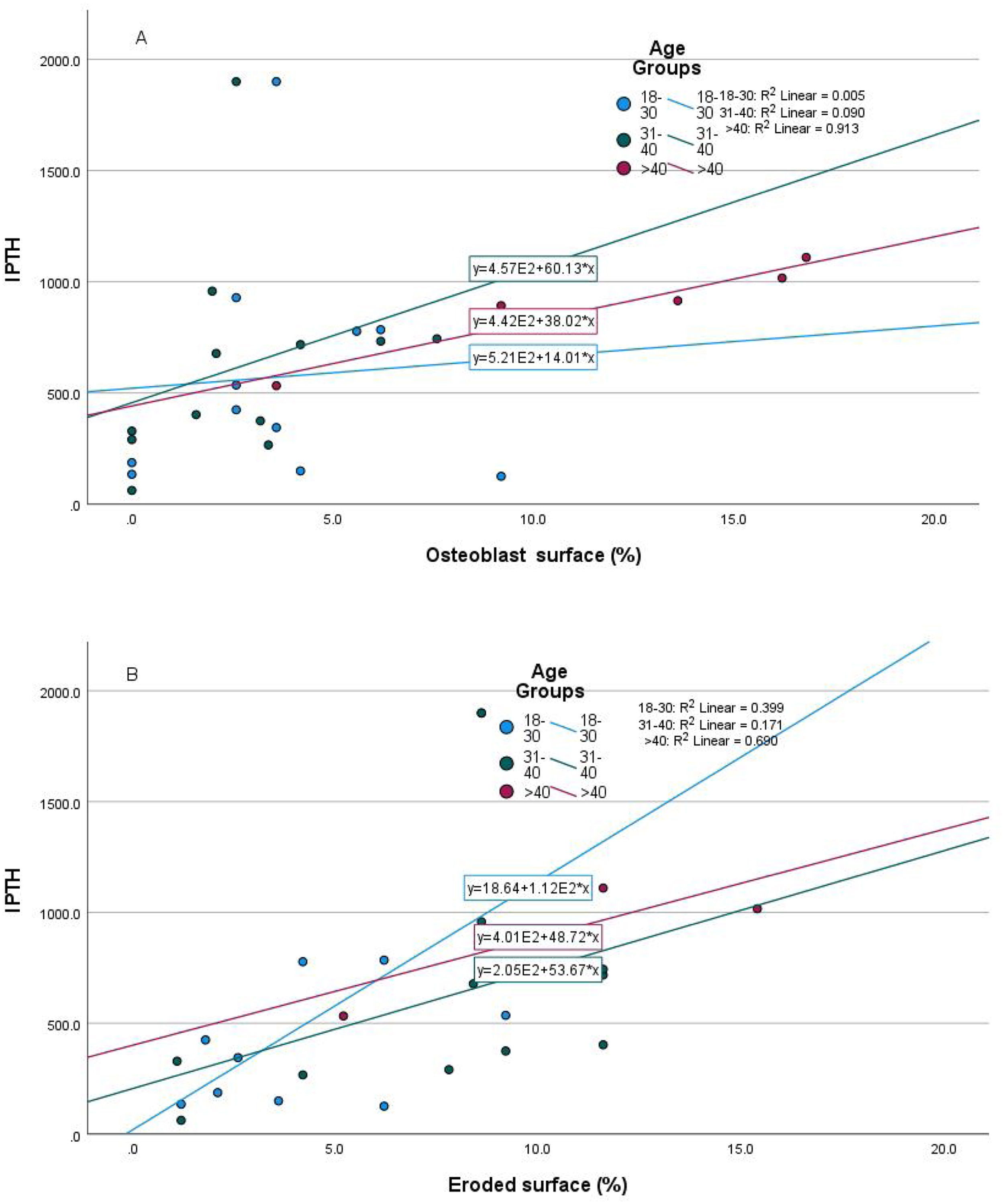
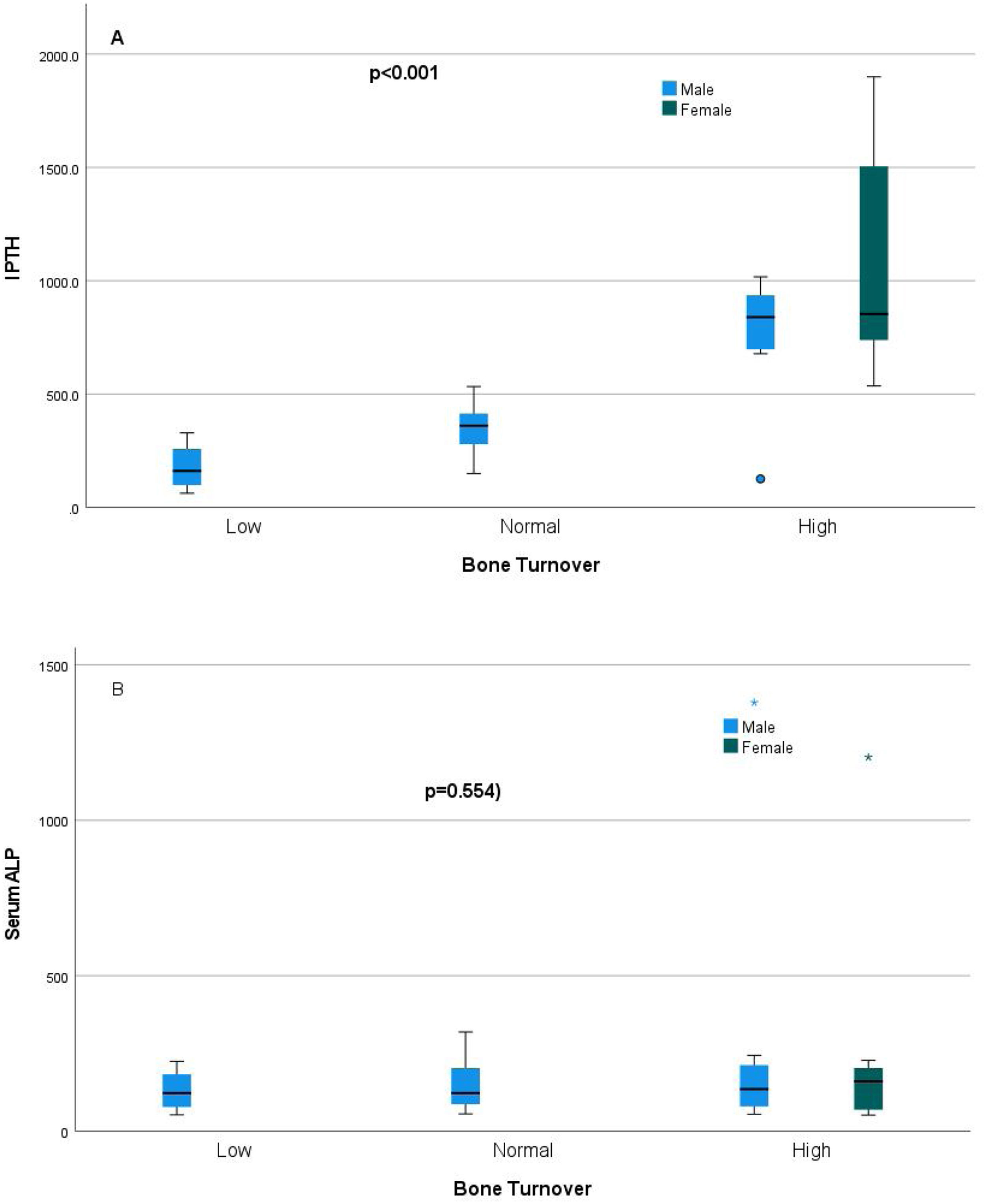
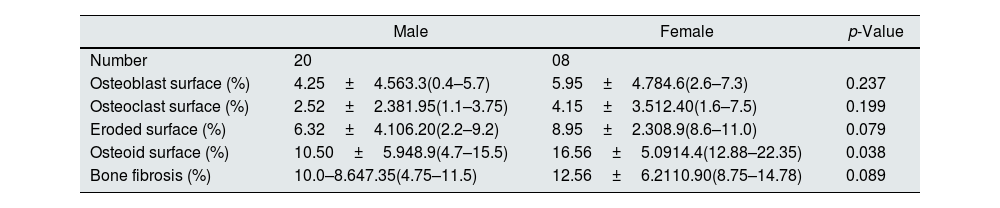
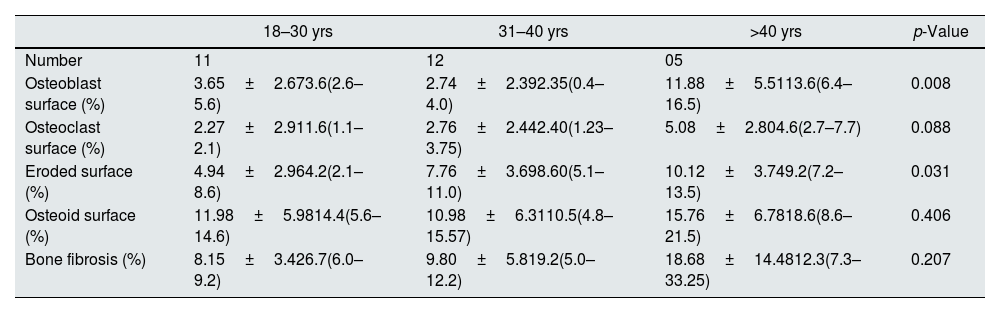
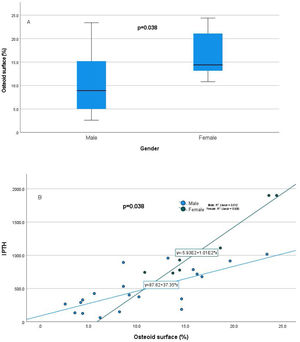
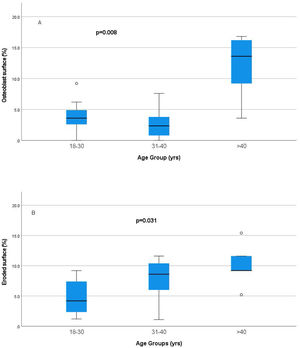
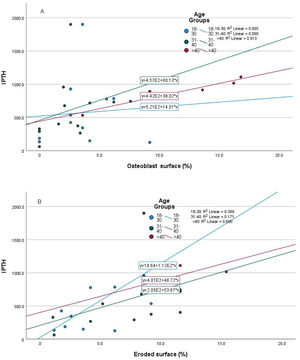
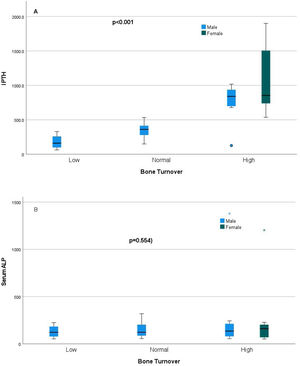
Relationship of static bone histomorphometric parameters with gender, age and bone turnoverAmong all the histological parameters, intact PTH was significantly associated with osteoclast surface, eroded bone surface, and osteoid surface. Fig. 1(A) highlights the combined impact of these parameters on the PTH level. Fig. 1(B) shows the relationship between bone fibrosis and serum alkaline phosphatase level. In static parameters, osteoid surface was significantly high in female compare to male (16.56±5.09 vs 10.50±5.94, p=0.031, Table 3 and Fig. 2A). Relationship of PTH and osteoid surface in both females and males is highlighted in Fig. 2B. Osteoblast and eroded surface were significantly high with increasing age (Table 4 and Fig. 3). In different age groups, relationship of PTH and static parameter (osteoblast and eroded surface) are highlighted in Fig. 4. Serum intact PTH was strongly associated with bone turnover state in our study (Fig. 5A, p<0.001). However, ALP was not significantly associated with bone turnover state (Fig. 5B).
Impact of gender on static histomorphometric parameters.
| Male | Female | p-Value | |
|---|---|---|---|
| Number | 20 | 08 | |
| Osteoblast surface (%) | 4.25±4.563.3(0.4–5.7) | 5.95±4.784.6(2.6–7.3) | 0.237 |
| Osteoclast surface (%) | 2.52±2.381.95(1.1–3.75) | 4.15±3.512.40(1.6–7.5) | 0.199 |
| Eroded surface (%) | 6.32±4.106.20(2.2–9.2) | 8.95±2.308.9(8.6–11.0) | 0.079 |
| Osteoid surface (%) | 10.50±5.948.9(4.7–15.5) | 16.56±5.0914.4(12.88–22.35) | 0.038 |
| Bone fibrosis (%) | 10.0–8.647.35(4.75–11.5) | 12.56±6.2110.90(8.75–14.78) | 0.089 |
Value in mean±SD, median (IQR), p<0.05 considered as significant.
Impact of age on static histomorphometric parameters.
| 18–30 yrs | 31–40 yrs | >40 yrs | p-Value | |
|---|---|---|---|---|
| Number | 11 | 12 | 05 | |
| Osteoblast surface (%) | 3.65±2.673.6(2.6–5.6) | 2.74±2.392.35(0.4–4.0) | 11.88±5.5113.6(6.4–16.5) | 0.008 |
| Osteoclast surface (%) | 2.27±2.911.6(1.1–2.1) | 2.76±2.442.40(1.23–3.75) | 5.08±2.804.6(2.7–7.7) | 0.088 |
| Eroded surface (%) | 4.94±2.964.2(2.1–8.6) | 7.76±3.698.60(5.1–11.0) | 10.12±3.749.2(7.2–13.5) | 0.031 |
| Osteoid surface (%) | 11.98±5.9814.4(5.6–14.6) | 10.98±6.3110.5(4.8–15.57) | 15.76±6.7818.6(8.6–21.5) | 0.406 |
| Bone fibrosis (%) | 8.15±3.426.7(6.0–9.2) | 9.80±5.819.2(5.0–12.2) | 18.68±14.4812.3(7.3–33.25) | 0.207 |
Value in mean±SD, median (IQR), p<0.05 considered as significant.
Our study estimated the relationship between commonly used laboratory parameters for assessing CKD-MBD and static bone histomorphometric parameters in dialysis patients. Patients were relatively young, and females had statistically high intact PTH levels. In female, estrogen receptors have been shown by parathyroid cells which favor parathyroid cell proliferation and parathyroid gland hyperplasia, even in uremic women.7 Its effect is independent of calcium, phosphorus, and vitamin D level. Differences in PTH levels have been reported in ESRD patients even with race.8 In the static histomorphometric parameters, only osteoid surface was significantly higher in females than in males (Fig. 2, p-value=0.038), which has a significant and independent relationship with intact PTH level (Table 2). In earlier studies, it has been reported that cancellous bone formation is stimulated by estrogen therapy, and it has been shown that it is due to the direct effect of estradiol on PTH secretion.9–11
Intact PTH level was significantly associated with bone formation parameters like osteoblast surface and osteoid surface. This has been shown in other studies also. PTH acts directly on early cells in the osteoblast lineage.12 PTH treatment decreases osteoblast apoptosis, increases osteoblast cell number and activates bone lining cells.13,14 However, whether persistent hyperparathyroidism, which is commonly seen in CKD state, affects lining cells like that of intermittent (pharmacologic) PTH treatment is an area that needs more research. In our study, male and female had different impact on static parameters in relation to PTH (Fig. 2B), especially osteoid surface was significantly different between the two groups (Table 3 and Fig. 2). Osteoblast and eroded surface had significant impact of age in our study (Table 4 and Figs. 3 and 4). In addition to bone formation parameters, PTH was significantly associated with bone resorption parameters like osteoclast surface, eroded surface, and bone fibrosis in our study. Hyperparathyroidism (both continuous and intermittent) increases bone resorption by osteoclasts. M-CSF and RANKL are the two major cytokines that drive osteoclast differentiation and function. The expression of both of these cytokines is increased by PTH.12 However, in our study, we did not measure these cytokines.
Two recently published studies, one by Salma et al. and another by Jorgensen et al., measured the predictability of static bone histomorphometric parameters for the high and low turnover state. The first study had a limited number of bone biopsies (n=43) and categorized patients by BFR alone, concluding that static parameters were not useful in predicting bone turnover.15 However, the second study by Jorgensen et al., which was adequately powered and had large total biopsies (n=205), used BFR and full histomorphometric analysis, concluded that static bone histomorphometry provides an acceptable alternative in predicting bone turnover in renal osteodystrophy in the absence of tetracycline level.16 In our study, we used static bone histomorphometric parameters to predict bone turnover.
Intact PTH, independently associated with most of these parameters, is a promising biomarker for the bone turnover state in our study. Even for bone turnover state, intact PTH is promosing biomarker (Fig. 5A, p<0.001). In a study by Sprague et al., PTH was considered as a good biomarker for low and high turnover state.17 Serum total ALP is also commonly used in clinical practice and is an important hydrolase enzyme related to the osteoblastic activity (especially bone-specific). Many studies have highlighted its role in patients with fibrous bone dysplasia (abnormal fibro-osseous condition).18 However, more studies must confirm its role in bone fibrosis in CKD patients (Fig. 2). It is not a promising biomarker of a low or high turnover state in our study (Fig. 5B). In adults with normal liver function, approximately 50% of the total AP activity in serum is derived from the liver, whereas 50% arises from bone hence total serum ALP is not a good biomarker for bone turnover; bone-specific ALP is more beneficial.19 However, in our study, we had not measured bone-specific ALP.
Our study has many limitations. Firstly, we did not use dynamic studies to compare our results. Bone formation rates can be different in different populations or races. Hence validation of the cut-off needs to be replicated with dynamic studies in our patients’ population. Secondly, we used freely available NIH validate software rather than live histological interpretation. The semi-quantitative methods of estimating bone histomorphometric parameters that we used in our study without dynamic studies are debatable and has potential to affect our result. Third, the sample size of our study was small; more studies with large sample sizes with dynamic studies are required to validate our findings. Fourth, we did not use bone-specific ALP (bs-ALP), a much more robust biomarker than total ALP for bone turnover assessment, and reported to be complementary to intact PTH in predicting the turnover state of renal osteodystrophy.
ConclusionOur study highlighted association of gender, age, intact PTH and ALP with static bone histomorphometric parameters in hemodialysis patients. Intact PTH was strongly associated with bone formation and bone resorption parameters and a reliable biomarker in predicting bone turnover state compare to total serum ALP. Further research with dynamic studies is required to validate our finding.
Conflict of interestWe declare no conflicting interests.