Klotho is found in two forms: a transmembrane form and a soluble form (s-Klotho). In order to be excreted, s-Klotho, that is too large to be filtered, will probably reach the proximal convoluted tubule by a transcytosis process. The aim of our study was to show the relationship between the levels of s-Klotho and tubular injury in patients with diabetic kidney disease (DKD), using as tubular injury marker the kidney injury molecule-1 (KIM-1).
MethodsOur study included 63 DKD patients (stages 1–5, mean eGFR 65.15±32.45ml/min) with a mean age 58.13±12 years. In all patients we determined serum levels of: KIM-1 and s-Klotho using ELISA, urinary albumin/creatinine ratio (UACR) and reduction in the estimated glomerular filtration rate (eGFR) per year.
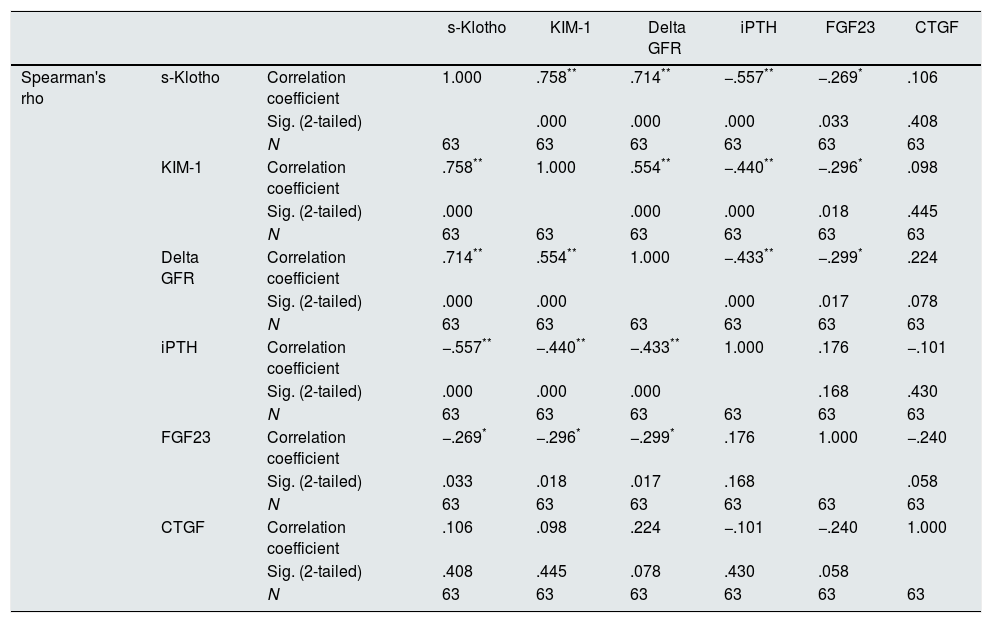
ResultsWe found a strong statistically significant correlation of s-Klotho with the rate of reduction of eGFR/year (r=0.714, p=0.0004) and with the tubular injury marker KIM-1 (r=0.758, p=0.005) and strong correlations of UACR with the rate of reduction of eGFR/year (r=0.53, p<0.01), KIM-1 (r=0.49, p<0.05) and s-Klotho (r=0.52, p<0.01).
ConclusionDespite previous published data, that shows a decrease of s-Klotho in chronic kidney disease, in our study the rapid annual decline of kidney function but not the level of eGFR was associated with increased s-Klotho. A possible explanation could be a more severe proximal tubule injury that could lead to a reduction of tubular excretion of s-Klotho as suggested by the correlation of s-Klotho levels with the serum levels of KIM-1.
Klotho se encuentra en el organismo en dos formas: una forma transmembranaria y una forma soluble (s-Klotho). Para excretarse s-Klotho, que es demasiado grande para ser filtrado, llegará en el túbulo contorneado proximal por un proceso de transcitosis. El objetivo del presente estudio es indicar la relación entre el nivel de s-Klotho y lesión tubular en los pacientes con la enfermedad renal diabética (DKD), utilizando como marcador de lesión tubular renal kidney injury molecule-1 (KIM-1).
MétodosNuestro estudio incluye 63 pacientes con DKD (etapas 1-5, eGFR medio 65,15 +/− 32,45 ml/min) con una edad media de 58,13 +/− 12 años. En todos los pacientes hemos determinado el nivel sérico de: KIM-1 y s-Klotho utilizando el método ELISA, coeficiente albúmina/creatinina urinaria (UACR) y la reducción de la tasa de filtración glomerular estimada (eGFR) al año.
ResultadosHemos encontrado una correlación fuerte significativa desde el punto de vista estadístico de s-Klotho con una tasa de reducción de eGFR/año (r = 0,714, p = 0,0004) y con el marcador de lesión tubular KIM-1 (r = 0,758, p = 0,005) y una fuerte correlación de UACR con una tasa de reducción de eGFR/año (r = 0,53, p < 0,01), KIM-1 (r = 0,49, p < 0,05) y s-Klotho (r = 0,52, p < 0,01).
ConclusionesA pesar de los datos publicados anteriormente en la literatura, que demuestran una reducción de s-Klotho en la enfermedad crónica de riñones, en nuestro estudio, la disminución rápida anual de la función renal y no el nivel de eGFR se correlaciona con el crecimiento de s-Klotho. Una posible explicación podría ser una lesión tubular proximal más grave que podría llevar a la reducción de la excreción tubular de s-Klotho, sugerida por la correlación de s-Klotho con el nivel sérico de KIM-1.
The identification of Klotho gene was important because it encodes a single-pass transmembrane co-receptor protein, expressed mainly (but not only) in the kidney, involved in the antiaging process. The Klotho protein is composed of a large extracellular and a small intracellular domain.1
The Klotho gene knock-out mice expresses a phenotype of premature aging: shortened life span, growth retardation, hypogonadism, skin and muscle atrophies, vascular calcification, cognition impairment, motor neuron degeneration and others.2 These actions are associated with phosphate balance disorders since transmembrane klotho acts as a co-receptor for FGF23, a hormone involved in phosphate reabsorbtion at the proximal tubule level and inhibition of the synthesis of active vitamin D.3
The extracellular domain of Klotho, after proteolytic cleavage, is released in the extracellular space as soluble Klotho (s-Klotho), identifiable in blood, urine, cerebrospinal fluid. s-Klotho exerts a phosphaturic effect, independent of FGF23 by inactivating the Na-Pi-2a transporter at the proximal tubule level. Beside the phosphaturic effect, soluble klotho has also other endocrine effects, such as inhibition of insulin-like growth factor (IGF)-1 receptors, inhibition of TGF beta mediated fibrogenesis, cytoprotection among others.4
The kidney is the main contributor to s-klotho production, and is also the major organ where s-klotho is retrieved from the circulation probably through tubular transcytosis.5 The question is how does chronic kidney disease (CKD) influence the level and functions of s-Klotho?
The aim of our study was to assess the level of s-Klotho in patients with diabetic kidney disease (DKD), the leading cause of end-stage renal disease,6 in relation with the rate of renal function decline. It is known that part of the patients with DKD are nonalbuminuric, suggesting that partly the decline of eGFR in diabetic patients is due to tubulo-interstitial injury7 and therefore we have studied the relationship of s-klotho with markers of tubular damage and with markers of interstitial fibrosis.
MethodsStudy populationThe study was performed in the University Nephrology Clinic of the “Pius Branzeu” Emergency County Hospital Timisoara, Romania. After signing an informed consent, we included 63 patients (30 female, 33 male) with a mean age of 58.13±12 years. All of the patients have been previously diagnosed with DKD (stages G1–G5) in the Diabetes and Metabolic Diseases Clinic, according to the criteria of the American Diabetes Association as the presence of an eGFR <60ml/min/1.73m2 or the presence of albuminuria >30mg/g creatinine.8 All patients were already treated with either ACE inhibitors or AII receptor blockers. All the patients were at their first referral to a nephrologist, thus none of them was previously diagnosed with CKD mineral bone disorder and none treated with vitamin D or phosphate binders. Patients undergoing renal replacement therapies have not been included. Demographic information, medical history (including duration of diabetes mellitus and history of eGFR in the past 12 months) were retrieved from the GP's and hospital data base in all cases.
Biochemical analysisIn every fasting patient after signing the informed consent spot urine and blood specimens were collected. We obtained the following parameters: serum creatinine (mg/dl), serum calcium (mg/dl), serum phosphorus (mg/dl), iPTH (pg/ml) and spot urinary albumin creatinine ratio (UACR – mg/g creatinine).
Serum creatinine has been performed using the Dimension Chem I Calibrator, traceable to the National Institute of Standards and Technology (NIST) Standard Reference Material (SRM) 914.
Estimated glomerular filtration rate (eGFR) was obtained in every patient using the CKD Epidemiology Collaboration equation (CKD-Epi), and the CKD stage was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines.9 We calculated the reduction of eGFR as the difference between current eGFR and previous eGFR (12 months earlier, obtained from the patient's files) divided through previous eGFR multiplied with 100, and we obtained the percentage of eGFR decrease.
ELISA techniqueBlood samples collected from the patients on the day of admission were assessed using ELISA technique in order to obtain serum levels of: soluble klotho, kidney injury molecule-1 (KIM-1), connective tissue growth factor (CTGF), fibroblast growth factor 23 (FGF23). We used the following commercially available antigen laboratory kits:
- •
Anti-Klotho antibody. The kit from Abbexa™ is based on sandwich enzyme-linked immune-sorbent assay technology. An horseradish peroxidase (HRP) conjugated anti-Klotho antibody is used as detection antibody. Tetramethyl-benzidin (TMB) substrate was used to visualize HRP enzymatic reaction.
- •
Boster's human KIM1 ELISA Kit, based on standard sandwich enzyme-linked immune-sorbent assay technology, that is a monoclonal antibody from mouse specific for KIM-1.
- •
OmniKine™ Human CTGF ELISA Kit, contains the components necessary for quantitative determination of natural or recombinant Human CTGF concentrations. This particular immunoassay utilizes the quantitative technique of a “Sandwich” Enzyme-Linked Immunosorbent Assay (ELISA) where the target protein (antigen) is bound in a “sandwich” format by the primary capture antibodies coated to each well-bottom and the secondary detection antibodies added subsequently by the investigator.
- •
Human FGF23 ELISA Kit (Aviscera Bioscience Inc.) contains the necessary components required for the quantitative measurement of recombinant and/or natural human FGF23 from serum and plasma in a sandwich ELISA format.
Data were collected and analysed using the SPSS v.17 software suite (SPSS Inc., Chicago, IL, USA) and are presented as mean±standard deviations for continuous variables with Gaussian distribution, median (interquartile range) for continuous variables without Gaussian distribution, or percentages for categorical variables. To assess the significance of the differences between groups, the Student t-test (means, Gaussian populations), Mann–Whitney U test (medians, non-Gaussian populations) and Chi-square (proportions) were used. Continuous variable distributions were tested for normality using Shapiro–Wilk test, and for equality of variances using Levene's test. The strength of the association between continuous variables was evaluated using Spearman's correlation coefficient. Since the primary hypothesis was to evaluate a possible relationship between s-Klotho and the amplitude of renal function decrease (an outcome which may be influenced by several other factors), besides the bivariate correlation between s-Klotho and ΔeGFR a multivariate regression model was built to evaluate the association between the two components, adjusted for confounding factors.
A p value of <0.05 was considered as the threshold for statistical significance.
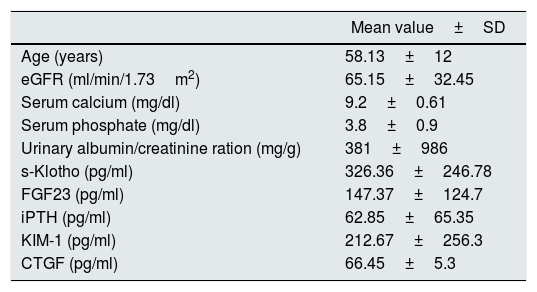
ResultsThe studied group baseline characteristics are presented in Table 1.
The baseline characteristics of the included patients.
| Mean value±SD | |
|---|---|
| Age (years) | 58.13±12 |
| eGFR (ml/min/1.73m2) | 65.15±32.45 |
| Serum calcium (mg/dl) | 9.2±0.61 |
| Serum phosphate (mg/dl) | 3.8±0.9 |
| Urinary albumin/creatinine ration (mg/g) | 381±986 |
| s-Klotho (pg/ml) | 326.36±246.78 |
| FGF23 (pg/ml) | 147.37±124.7 |
| iPTH (pg/ml) | 62.85±65.35 |
| KIM-1 (pg/ml) | 212.67±256.3 |
| CTGF (pg/ml) | 66.45±5.3 |
In our cohort we found a mean reduction of eGFR over the previous 12 months of 19.23±15.01% corresponding to 11.6±8.24ml/min/1.73m2/year.
We divided the studied subjects into those with eGFR >60ml/min/1.73m2 – 34 patients (this group included patients with DKD stage 1 and 2) and 29 patients with eGFR <60ml/min/1.73m2 (this group included patients with DKD stages 3–5 ND).
The main correlations found comparing the studied variables are presented in Table 2.
Correlations found between the studied variables (S-Klotho, KIM-1, rate of reduction of eGFR/year – Delta GFR, iPTH, FGF23, CTGF).
| s-Klotho | KIM-1 | Delta GFR | iPTH | FGF23 | CTGF | |||
|---|---|---|---|---|---|---|---|---|
| Spearman's rho | s-Klotho | Correlation coefficient | 1.000 | .758** | .714** | −.557** | −.269* | .106 |
| Sig. (2-tailed) | .000 | .000 | .000 | .033 | .408 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 | ||
| KIM-1 | Correlation coefficient | .758** | 1.000 | .554** | −.440** | −.296* | .098 | |
| Sig. (2-tailed) | .000 | .000 | .000 | .018 | .445 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 | ||
| Delta GFR | Correlation coefficient | .714** | .554** | 1.000 | −.433** | −.299* | .224 | |
| Sig. (2-tailed) | .000 | .000 | .000 | .017 | .078 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 | ||
| iPTH | Correlation coefficient | −.557** | −.440** | −.433** | 1.000 | .176 | −.101 | |
| Sig. (2-tailed) | .000 | .000 | .000 | .168 | .430 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 | ||
| FGF23 | Correlation coefficient | −.269* | −.296* | −.299* | .176 | 1.000 | −.240 | |
| Sig. (2-tailed) | .033 | .018 | .017 | .168 | .058 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 | ||
| CTGF | Correlation coefficient | .106 | .098 | .224 | −.101 | −.240 | 1.000 | |
| Sig. (2-tailed) | .408 | .445 | .078 | .430 | .058 | |||
| N | 63 | 63 | 63 | 63 | 63 | 63 |
** Correlation is significant at the 0.01 level (2-tailed).
* Correlation is significant at the 0.05 level (2-tailed).
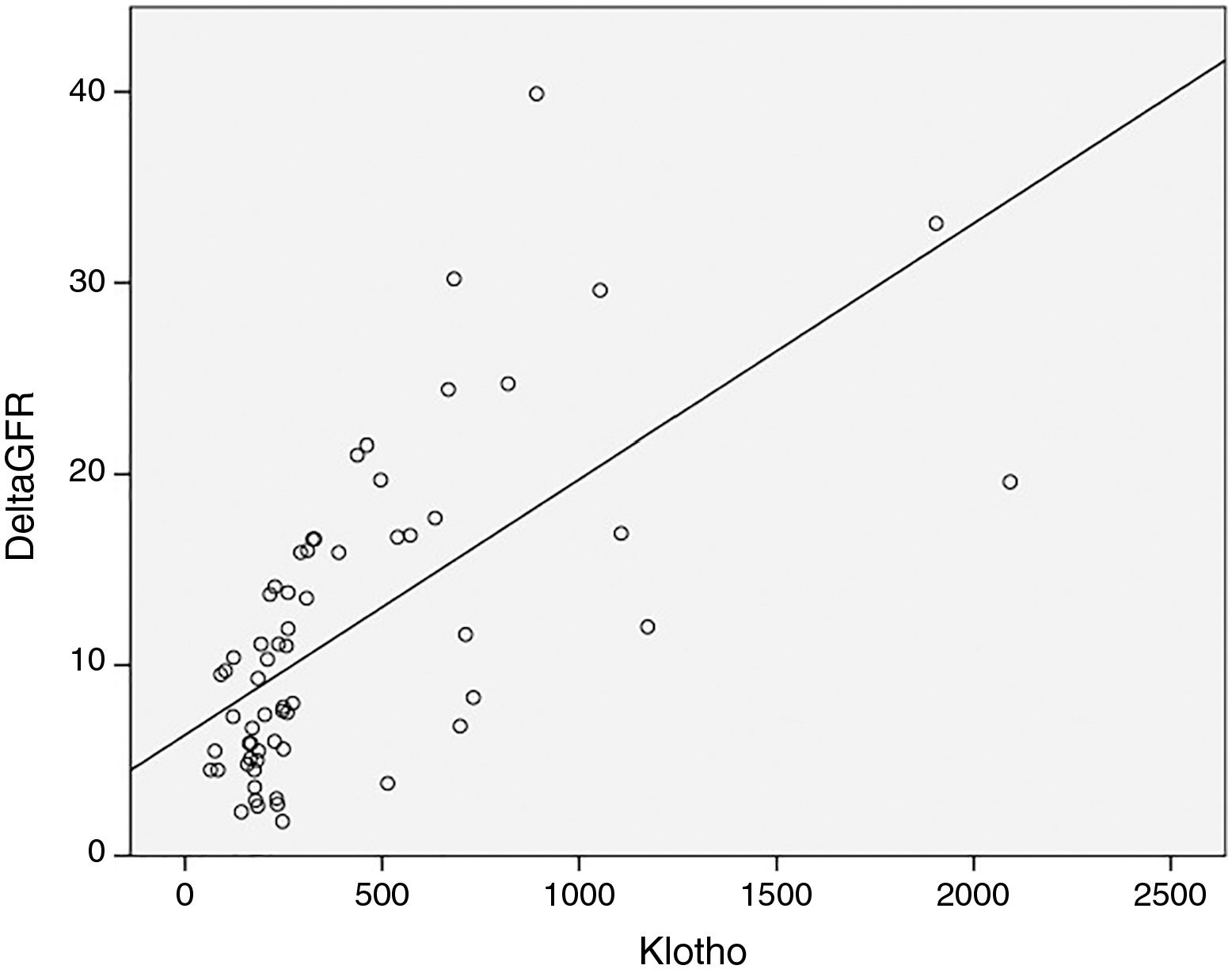
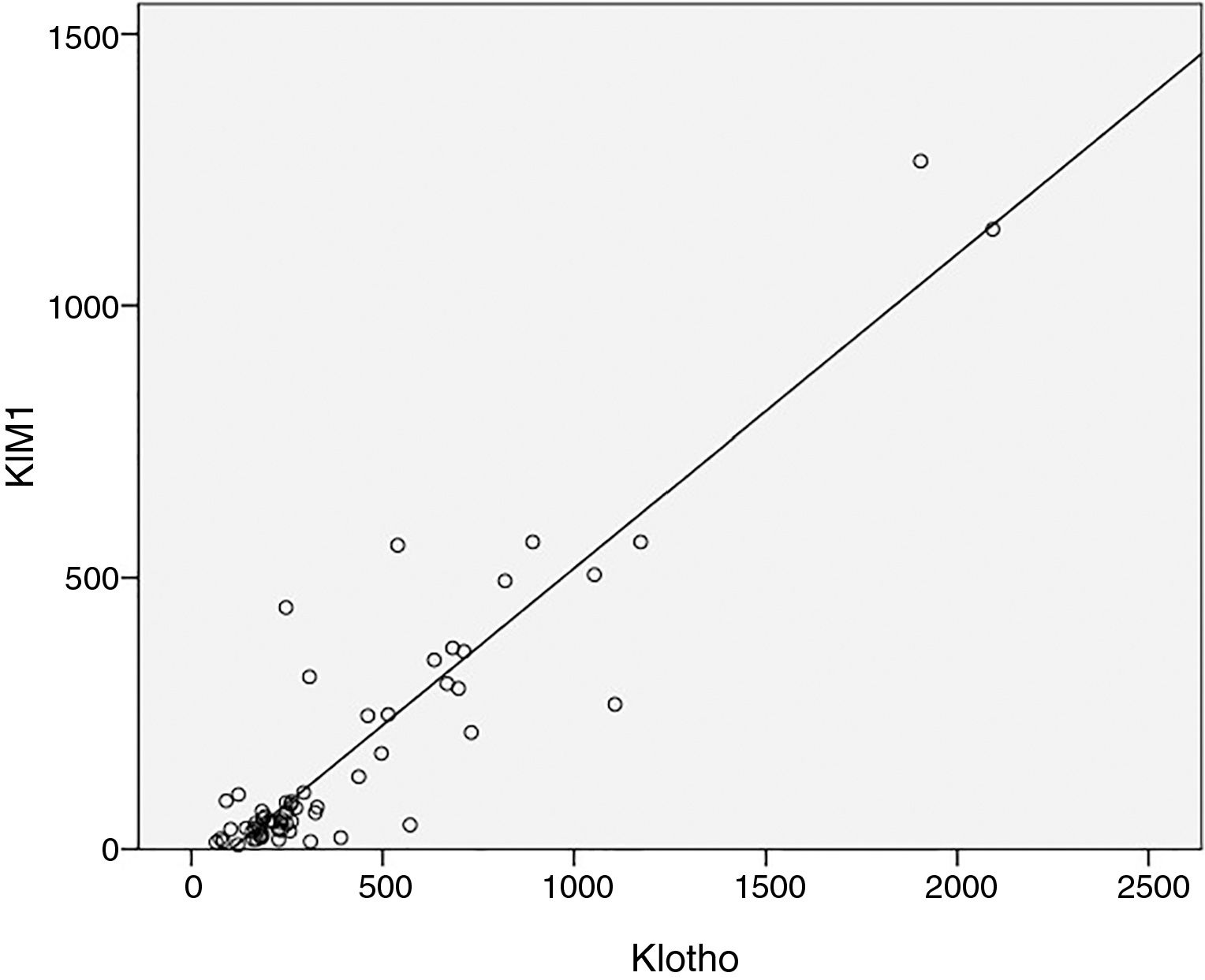
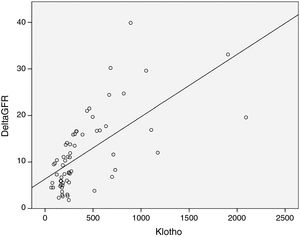
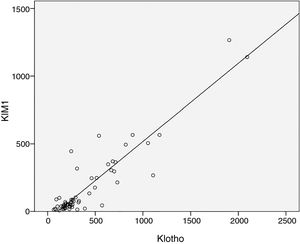
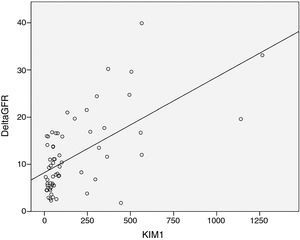
As expected s-Klotho correlated negatively with the age of the patients (r=−0.31, p=0.014). The s-Klotho level did not correlate with eGFR, however, on average it was higher (but not significantly) in patients with eGFR below 60ml/min/1.73m2. We found a strong correlation of s-Klotho with the rate of reduction of eGFR/year (r=0.714, p=0.0004) and with the tubular injury marker KIM-1 (r=0.758, p=0.005) (Figs. 1 and 2).
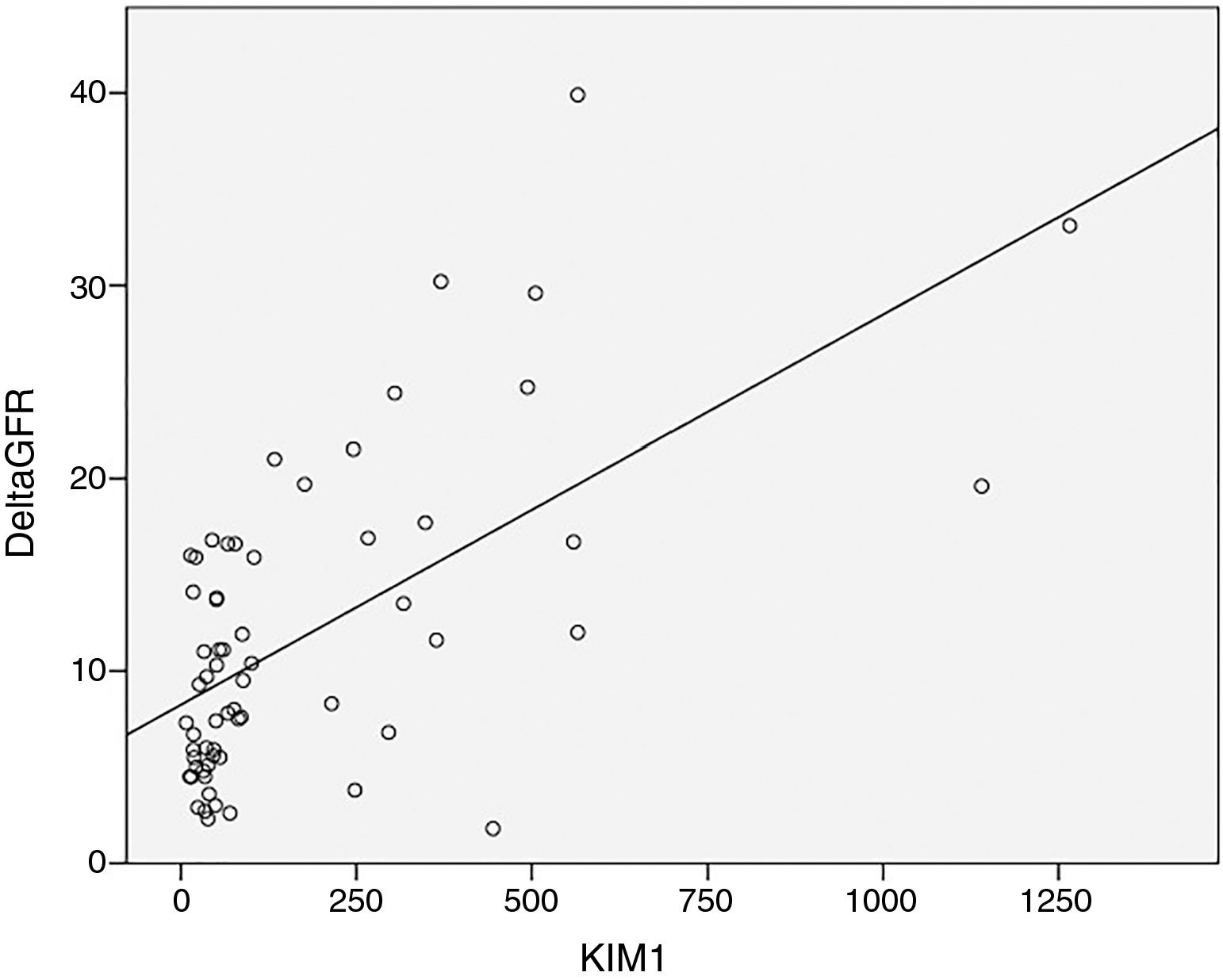
KIM-1 levels showed a similar pattern: no correlation with eGFR but a strong correlation with the rate of reduction of eGFR/year (r=0.554, p=0.0004) (Fig. 3).
We found strong correlations of urinary albumin/creatinine ratio with the rate of reduction of eGFR/year (r=0.53, p<0.01), KIM-1 (r=0.49, p<0.05) and s-Klotho (r=0.52, p<0.01).
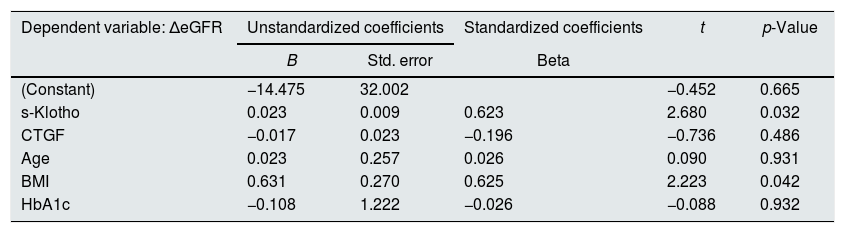
To evaluate the main hypothesis of the study (the association between s-Klotho and the amplitude of renal function decline), considering that the renal function decline may be influenced by several other factors, to adjust the relationship for confounding factors we built a multivariate regression model, having as outcome the eGFR rate of decline and predictors: s-Klotho, CTGF, patient's age, HbA1c and BMI (Table 3).
Multivariate regression analysis of the rate of reduction of eGFR/year.
| Dependent variable: ΔeGFR | Unstandardized coefficients | Standardized coefficients | t | p-Value | |
|---|---|---|---|---|---|
| B | Std. error | Beta | |||
| (Constant) | −14.475 | 32.002 | −0.452 | 0.665 | |
| s-Klotho | 0.023 | 0.009 | 0.623 | 2.680 | 0.032 |
| CTGF | −0.017 | 0.023 | −0.196 | −0.736 | 0.486 |
| Age | 0.023 | 0.257 | 0.026 | 0.090 | 0.931 |
| BMI | 0.631 | 0.270 | 0.625 | 2.223 | 0.042 |
| HbA1c | −0.108 | 1.222 | −0.026 | −0.088 | 0.932 |
The results of the multivariate regression model are pointing that the values of s-Klotho are significantly associated with the decline of the renal function both in the univariate model as well as after adjusting for possible confounding factors. According to the resulted model, in our scenario, after confounding adjustments we observed that for one increase with a standard deviation in the s-Klotho value we may expect an augmentation of the renal function decline (as evaluated using ΔeGFR) with 0.623 standard deviations.
This model revealed also that a significant predictor of the renal function decline in this cohort of patients was the BMI value (an increased BMI being associated in the multivariate model with an increased rate of decline in the renal function).
DiscussionIn the recent years a high number of research papers are addressing Klotho genetics, functions and possible use for early diagnosis in CKD, prognostic assessment in CKD and associated cardiovascular disease (CVD). The s-Klotho level was proposed as a biomarker for early stages of CKD.10 Higher soluble klotho levels proved to be independently associated with a lower risk for decline of the kidney function,11 while reduced levels were associated with higher cardiovascular and all-cause mortality.12 However contradictory results have also been published at least concerning mortality in CKD patients.13,14. s-Klotho was also proposed as a possible therapeutic measure.15 In mice with CKD restoring the Klotho level induced negative phosphate balance through inhibition of renal phosphate transporters and prevented vascular calcification by inhibiting phosphate uptake.16 A recent experimental study showed that recombinant Klotho protein is effective in preventing or retarding acute kidney injury progression to CKD in mice.17
The highest expression of membrane Klotho was evidenced at the kidney level and with the progression of CKD its level is decreasing.18 Animal studies have shown that the kidney is the main source of s-Klotho.19 Thus, because presumably the majority of s-Klotho is derived from renal membrane Klotho, renal function decline should be associated with a decrease of s-Klotho. Studies performed in patients with CKD stages 1–5 HD, show a decrease of s-Klotho compared to normal subjects, the decrease being more important with the progression of CKD.20,21 Even in the absence of a deterioration of renal function after nephrectomy in living renal donors, a decrease of s-Klotho has been observed.22
Despite the data presented before, in our study patients with eGFR below 60ml/min, had on average higher s-Klotho levels as compared to those with higher eGFR (without reaching statistical significance). Instead we found a strong statistically correlation between the level of s-Klotho and the rate of decrease of eGFR/year (r=0.714, p=0.0004), thus patients that showed a faster progression of CKD had higher levels of s-Klotho.
The differences reported in the level of s-Klotho by different authors, are probably explained by an assay-related variance,4 however there is data, supporting our observations, showing an increase of s-Klotho in CKD23–25 or in acute kidney injury (AKI).26 The question is why s-Klotho levels are not always decreasing with the progression of kidney disease, following the pattern of membrane klotho?
It is known that in subjects with CKD the expression of renal Klotho is severely impaired, reaching levels as far as 5% of those encountered in subjects without renal impairment,2 but serum levels of s-Klotho in patients with CKD, are only modestly reduced compared to values reported in healthy adults.27
Thus the circulating level of Klotho does not probably reflect the level of tissue Klotho, especially in patients with CKD.8 This could be due to the extrarenal origin of s-Klotho (parathyroids,28 choroid processes, vascular wall).
The discrepancies between the higher level of circulating s-Klotho and decreased renal function could also be attributed to a decreased excretion. The route of excretion of s-klotho is unknown. An indirect proof of the presence of s-Klotho in urine is the independent phosphaturic effect exerted at tubular level. Soluble Klotho acts from the urinary luminal side as an autocrine or paracrine enzyme to regulate transporters and ion channels, the phosphaturic effect being obtained by modifying the Na+-phosphate cotransporter NaPi-2a. This effect is independent of FGF23.29 The presence of s-Klotho in the urine but not in the Bowman's space was evidenced in an animal model after injecting Klotho i.v. supporting the fact that s-Klotho is too large (130kDa) to be filtered through the glomerular barrier.30 Most probably to reach the lumen of the proximal tubules, s-Klotho undergoes a transcytosis process, at the level of the proximal tubules.5
In case of a nonfunctioning kidney it is unclear what happens to the production and clearance of Klotho. Extra-renal Klotho production might be low or undetectable in normal conditions, or even in acute loss of renal function, but could possibly be stimulated in chronic Klotho deficiency. Another interesting finding, supporting the role of renal tubules in the clearance of s-Klotho in the failing kidney, was evidenced in peritoneal dialysis patients. Serum s-Klotho levels turned out to be negatively correlated with the urine output rather than with the remaining kidney function expressed by eGFR.31
Considering the hypothesis of the tubular transcytosis of s-Klotho, and the negative correlation of the serum level of s-Klotho with urine output, we raise the question of the relationship between s-Klotho and tubular injury, also because tubular basement membrane thickening and tubular atrophy belong to the classical interstitial pathological changes in DKD.7
Kidney injury molecule-1 (KIM-1) is a transmembrane tubular protein expressed in response to tubular injury, being a sensitive marker of acute kidney injury. The increase of urinary KIM-1, due to its link to tubular atrophy could also be used to predict progression of CKD,32 but the question is if plasma levels of KIM-1 are useful as well. In a study performed by Alter et al. proximal tubular injury due to onset diabetes in rats leads to elevated plasma KIM-1 levels.33 Plasma KIM-1 is considered the first blood biomarker that specifically reflects injury to the proximal tubule of the kidney.34 In patients with diabetes, increased plasma KIM-1 levels are associated to an increased risk of renal function decline.35
We observed in our study that plasma KIM-1 is higher in DKD patients with eGFR <60ml/min (239.6 vs. 173.3), however without statistical significance (p=0.5). But we found a strong positive correlation of plasma KIM-1 with the rate of reduction of eGFR/year, showing thus a correlation between chronic tubular injury and renal function decline. We have also found that increased plasma KIM-1 levels are associated with increased s-Klotho levels, suggesting the hypothesis that more severe proximal tubular injury is associated to increased levels of s-Klotho. So it is possible that the more rapid progression of DKD in our patients is related to more severe proximal tubule injury than other mechanisms (at least in our cohort of patients).
Another interesting relationship that we have found is the direct correlation of urinary albumin/creatinine ratio with KIM-1 levels and s-klotho. This could emphasize the above hypothesis, that lesion of the proximal tubular cells is associated also to an increase in albuminuria, besides the increase of s-Klotho. The relationship between tubular injury and albuminuria has been explained by a hypothetical “retrieval pathway” of filtered albumin, that returns albumin through a transcytosis process to the peritubular blood supply.36 Injury at the tubular level could thus lead to an increase in albuminuria, like we have observed in our cohort, and it could also explain the correlation observed between albuminuria and s-Klotho.
One of the pleiotropic effects of s-klotho is the anti-fibrosis effect .37 Because this effect is linked to the potential benefits of restoring klotho levels, we have tried to assess if there is a relationship between s-klotho levels and a biomarker of fibrogenesis. CTGF is suggested to be a contributor to the tubulointerstitial fibrosis that accompanies proteinuria in CKD.38 However, in our patients we could not find any statistically significant correlation between s-Klotho and CTGF, and therefore this issue needs more extensive study in the future. Another important aspect is the relationship between s-Klotho and age, because it is known that Klotho was discovered as an aging gene. In our patients we found a decrease of s-Klotho in older patients. Other authors that measured s-Klotho showed higher levels in children,39 but a weaker40 or no relationship with age in adults.41
A particularity of the studied patient group is the rather high rate of renal function decline, higher compared to the decline reported in the literature for DKD.42 According to the KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease we could define our results as a rapid decline of the renal function (>5mlmin/year).43 One explanation could be that our included patients were all recent referrals to the nephrologist, without previous intervention strategies of delaying renal disease progression.
An important limitation of the study is related to the technical difficulty of the s-Klotho measurement, resulting from the lack of standardization between the different commercially available kits, and also by the fact that a cross-reactivity with other analytes cannot be excluded.44 To the limitations of our study we would count the relatively small number of patients and the fact that data is not analyzed prospectively; the small sample size analyzed may lead to type II statistical errors by not having enough statistical power to reject the null hypothesis. Even if the sample size is small and the lack of statistical power may lead to some inferential impairments, we do not consider this a major drawback considering the exploratory and innovative character of the study; the results of associations, even some of them not being demonstrated as statistically significant may lead to novel, revolutionary approaches regarding the role of s-Klotho in chronic kidney disease. For a better assessment of the relationship with CKD-MBD markers the level of phosphaturia and also collecting data regarding the dietary phosphate intake would have been of help.
ConclusionsDespite previous published data, that shows a decrease of s-Klotho in chronic kidney disease, in our study the rapid annual decline of kidney function but not the level of eGFR was associated with increased s-Klotho levels. A possible explanation could be a more severe proximal tubule injury that could lead to a reduction of tubular excretion of s-Klotho as suggested by the correlation of s-Klotho levels with the serum levels of KIM-1. Future human studies are needed in order to reveal the relationship between tubular injury and s-Klotho in chronic kidney disease.
Ethical approvalAll procedures performed in this study were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number 183E-94) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
Conflict of interestThe authors have declared that no conflict of interest exists.
The study was supported by a research grant (PIII-C1-PCFI-2015/2016) of the University of Medicine and Pharmacy “Victor Babes” Timisoara, Romania.
Authors Flaviu Bob and Adalbert Schiller had an equal contribution to this study.